Randomization and Blinding (Masking)
Randomization
 Randomization is a method of allocating subjects in a clinical trial to treatment groups such that every subject has an equal chance of receiving any one of the treatments or interventions. This can be achieved by any fair method that assigns subjects in a completely unpredictable fashion. One could use the flip of a coin if there are only two treatment options, but more commonly a table of random numbers or computer-generated random numbers are used. Other methods, such as assigning subjects based on odd or even calendar date, can be "gamed" in a way that biases assignment.
Randomization is a method of allocating subjects in a clinical trial to treatment groups such that every subject has an equal chance of receiving any one of the treatments or interventions. This can be achieved by any fair method that assigns subjects in a completely unpredictable fashion. One could use the flip of a coin if there are only two treatment options, but more commonly a table of random numbers or computer-generated random numbers are used. Other methods, such as assigning subjects based on odd or even calendar date, can be "gamed" in a way that biases assignment.
If assignment is truly unpredictable, then there is no bias in assignment, and neither the subjects nor the investigators can influence assignment. In addition, randomization of a large number of subjects tends to result in groups that differ only in treatment and are comparable with respect to all other factors and characteristics that might influence the outcome. As a result, randomization is the best method for eliminating confounding.
Blinding
Blinded (or "masked") studies are those in which the subjects, and possibly the investigators as well, are unaware of which treatment the subject is receiving, e.g., active drug or placebo. Blinding is particularly important in drug trials when the study is assessing subjective outcomes, such as relief of pain or anxiety.
It isn't always possible to mask the treatments. For example, subjects randomly assigned to follow either a specific exercise regimen or continue their usual level of activity cannot be blinded.
- Single-blinded: the subjects are unaware of which group they have been assigned to.
- Double-blinded: Neither the subjects nor the investigators are aware of the treatment assignment until the end of the trial.
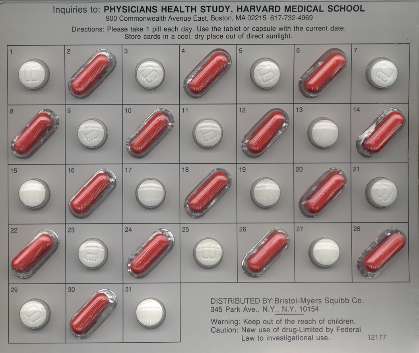
A placebo is an inert substance identical in appearance to the active treatment. Its purpose is to facilitate blinding by making the groups as similar as possible in the perception of treatment and to promote compliance. In the Physicians' Health Study participants were given a blister pack for each month (shown in the image below) that contained white tablets and red capsules that were taken on alternate days. The white tablets contained either 325 mg. of aspirin or an identical-looking inert substance; the red capsules contained either beta-carotene or an inert substance. The use of monthly blister packs also made it easier for participants to keep track of whether they had taken the correct pill each day.
It is not always ethical to use a placebo. If there is already a standard treatment or method of care, it would be unethical to withhold it. A new treatment should be compared to the standard therapy rather than to a placebo.
Example of Placebo Use to Achieve Blinding:
Glucosamine and chondroitin are naturally occurring substances that are structural components of the cartilage that lines our joints. Health food stores began selling supplements to people as a prevention (or treatment) for osteoarthritis despite a lack of evidence of their benefit in humans. Clegg and colleagues conducted a double-blind, randomized clinical trial in 1583 subjects with symptomatic osteoarthritis of the knee. Participants were randomly assigned to one of five treatment arms in order to test the efficacy of glucosamine and chondroitin. The primary outcome was greater than 20% decrease in total score on the WOMAC pain scale from baseline to week 24. Some of their results are shown in the table below.
| Pain relief >20% | Minimal Effect | Total # Subjects | |
|---|---|---|---|
| Placebo | 188 | 125 | 313 |
| Anti-inflammatory drug
|
223 | 95 | 318 |
| Glucosamine | 203 | 114 | 317 |
| Chondroitin | 208 | 110 | 318 |
| Glucosamine + Chondroitin | 211 | 106 | 317 |
Data from Clegg DO, et al.: Glucosamine, chondroitin sulfate, and the two
in combination for painful knee osteoarthritis. N Engl J Med 354:795, 2006.
Perhaps the most remarkable observation is the response in the group treated with the placebo which had a cumulative incidence of >20% pain relief of 60% (188/313 = 0.60 = 60%)! This is an example of the "placebo effect" in which patients who perceive they are being treated often report subjective improvement, even if the treatment has no effect. Placebos make the perception of treatment similar among groups and provide a reference group that takes into account the placebo effect. Note also that the group treated with glucosamine and chondroitin had only a slightly greater response rate of 67%.