The Physicians' Health Study on Aspirin
As early as the 1950s there were case series and small clinical trials suggesting that aspirin might be beneficial in preventing myocardial infarction (heart attack). However, the reduction in risk appeared to be modest, and the studies were too small to demonstrate a statistically significant benefit. Therefore, investigators at Harvard Medical School sought funding for a large phase 3 clinical trial.
Enrollment
In 1981, after receiving approval from the Institutional Review Board at Harvard Medical School, the investigators mailed invitation letters, consent forms, and enrollment questionnaires to all 261,248 registered male physicians in the US between 40 and 84 years old. (Phase 1 and phase 2 trials were unnecessary, because aspirin was a commonly used drug with known dosage range and known side effects.)

Questionnaires were returned by 112,528 physicians, but only 59,285 of those were willing to participate in the trial. Of those, 26,062 could not participate because they had one or more of the exclusion criteria:
- past myocardial infarction, stroke, or transient ischemic attack;
- cancer (except non-melanoma skin cancer);
- current renal or liver disease;
- peptic ulcer;
- gout;
- contraindication to or current use of either aspirin or beta-carotene.
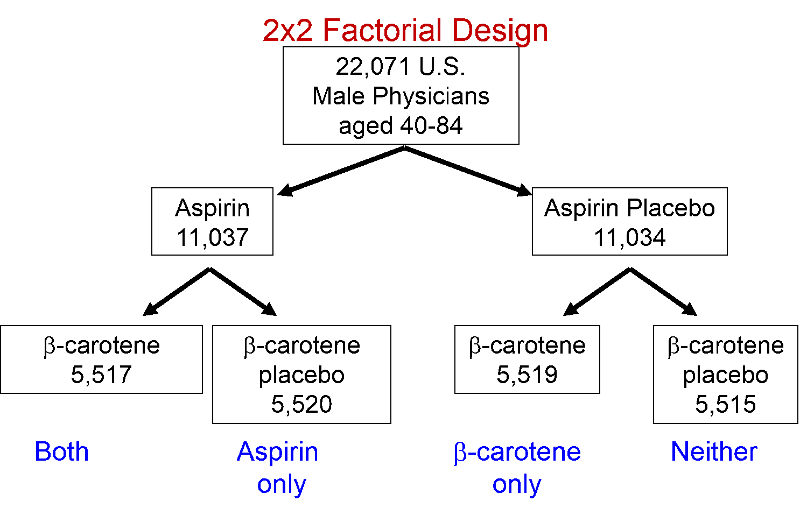
Informed consent was obtained from the 33,223 who were willing and eligible to participate. Since regular aspirin use has the potential to cause gastritis and bleeding problems, these physicians were enrolled in an 18-week run-in phase, during which all received active aspirin and placebo beta-carotene for 18 weeks. Some had unpleasant side effects, others decided not to participate, and some were excused because they didn't take the medications reliably. The remaining 22,071 men were then randomly assigned to one of four treatment groups.