Types of Intervention Studies
Therapeutic vs. Preventive
Clinical trials in individuals can be classified as either therapeutic or preventive, as in these examples:
Therapeutic Trials: New treatments are tested for the effectiveness in treating disease, e.g.,
- Does the drug herceptin improve survival in women already diagnosed with breast cancer?
- Does treatment with Tamiflu shorten the duration and improve survival in patients with bird flu?
Preventive Trials: Healthy or high-risk individuals are tested to determine whether a treatment prevents disease, e.g.,
- Does the drug tamoxifen prevent development of breast cancer in women who have a high risk of developing breast cancer?
- How effective is this year's influenza vaccine in preventing the flu?
- Does a Mediterranean diet reduce the incidence of cardiovascular disease?
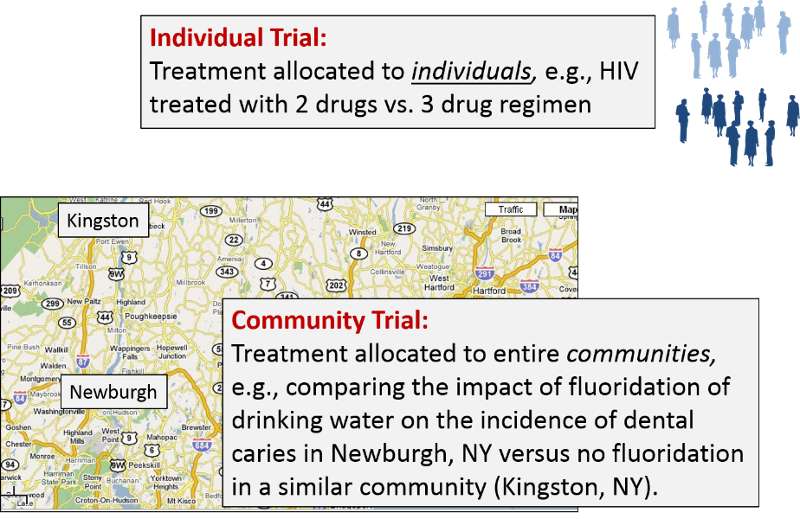
Individual Trials vs. Community Trials
Preventive measures can also be allocated on a community level – so-called community trials. A classic example is the Newburgh-Kingston Caries Fluoride Study which began in 1947. Fluoride was added to the water supply of Newburgh, NY, and the incidence of dental caries in Newburgh was then compared to the incidence in Kingston, NY, which did not receive fluoride. The trial demonstrated that addition of tiny amounts of fluoride to the water supply reduced dental caries by two thirds in children who began drinking fluoridated water within their first two years.
The key difference is that in community trials the treatments being studied are allocated not to individuals, but to entire communities.
Phases of Individual Therapeutic Trials
When most people hear reference to a clinical trial, they think of phase 3 trials in which large numbers of subjects are enrolled and randomly assigned to one of the treatment groups. However, phase 3 trials of new drugs with potentially harmful side effects are preceded by extensive studies in lab animals and by phase I and phase 2 trials in human volunteers.
Phase 1 Clinical Trials
If studies in animals suggest efficacy and safety, a phase 1 trial can be conducted in a small group (10-30) of human volunteers over 2-12 months, primarily to test for safety and to identify side effects, but also to get some information on effective dose.
Phase 2 Clinical Trials
Phase 2 clinical trials involve more volunteers than phase 1, and they typically last about two years. They usually involve two or more groups receiving different doses of the new drug in order to establish its therapeutic range of the drug, i.e., doses at which it is effective and has an acceptable level of side effects. If results suggest efficacy and safety, a phase 3 trial will be conducted.
Phase 3 Clinical Trials
Phase 3 trials are similar to prospective cohort studies in their design, except that the exposure of interest is a drug or some other intervention that is randomly assigned to the participants by the investigators. To facilitate this presentation of phase 3 trials we will focus on the first Physicians' Health Study, which began in 1981 in order to test the efficacy of aspirin in primary prevention of myocardial infarction. A second goal of the study was to evaluate the efficacy of beta-carotene in preventing cancer, but this discussion will focus on the aspirin component.