Introduction
The primary goal of observational studies, e.g., case-control studies and cohort studies, is to test hypotheses about the determinants of disease. In contrast, the goal of intervention studies is to test the efficacy of specific treatments or preventive measures by assigning individual subjects to one of two or more treatment or prevention options. Intervention studies often test the efficacy of drugs, but one might also use this design to test the efficacy of differing management strategies or regimens. There are two major types of intervention studies:
- Controlled clinical trials in which individual subjects are assigned to one or another of the competing interventions, or
- Community interventions, in which an intervention is assigned to an entire group.
In many respects the design of a clinical trial is analogous to a prospective cohort study, except that the investigators assign or allocate the exposure (treatment) under study.
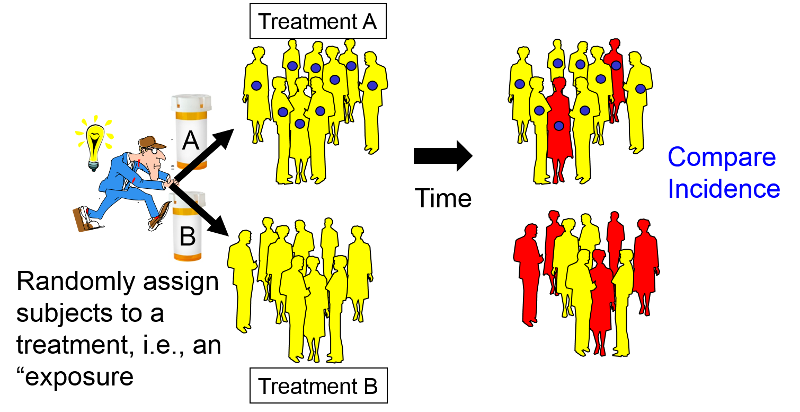
This provides clinical trials with a powerful advantage over observational studies, provided the assignment to a treatment group is done randomly with a sufficiently large sample size. Under these circumstances randomized clinical trials (RCTs) provide the best opportunity to control for confounding and avoid certain biases. Consequently, they provide the most effective way to detect small to moderate benefits of one treatment over another. However, in order to provide definitive answers, clinical trials must enroll a sufficient number of appropriate subjects and follow them for an adequate period of time. Consequently, clinical trials can be long and expensive.
Learning Objectives
After successfully completing this section, the student will be able to:
- Explain the distinguishing features of a clinical trial (intervention study).
- Discuss the two major potential advantages of intervention studies and their limitations.
- Differentiate between preventive, therapeutic, individual and community RCTs
- Briefly explain the differences among phase I, II, III, & IV clinical trials.
- Define randomization in the context of a clinical trial and give examples of appropriate methods of randomization.
- Explain why randomization is used.
- Explain how to determine whether randomization has been successful.
- Define blinding and explain the purpose of blinding.
- Distinguish between single and double blinding.
- Explain what the placebo effect is.
- Define the term "placebo" and explain why placebos are used.
- Explain when the use of a placebo is not appropriate and discuss alternative strategies.
- Explain why it is important to maintain high rates of follow-up in a prospective cohort study or a clinical trial.
- Explain why compliance is important and the effects of non-compliance.
- Define and distinguish between "intention to treat analysis" and an efficacy analysis.
- Define what a run-in phase is and explain its purpose.