Types of Intervention Studies
Individual versus Group (Community) Trials
- Most trials are conducted by allocating treatments or interventions to individual subjects, i.e., the treatment or intervention is allocated to individuals. For example, investigators recently compared the effectiveness of glucosamine and chondroitin and several other drugs in their ability to relieve symptoms of osteoarthritis. Subjects were randomly assigned to receive one of several possible treatments, and they were followed and assessed for pain relief and other measures.
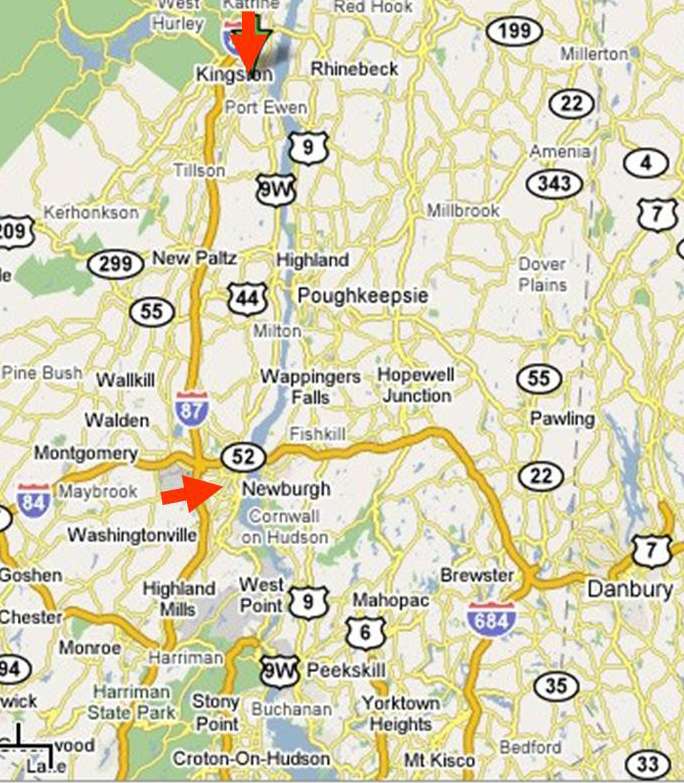
- In contrast, group trials allocate the intervention to groups of subjects. These types of trials are generally conducted when the intervention is inherently operates at a group-level (e.g., changing a law or policy) or because it would be difficult to give the intervention to some people in the group while withholding it from others. Group units might be families, schools, or medical practices. A well-known type of group trial is a community trial, in which the intervention is allocated therapy to entire communities or neighborhoods. In the 1940s the effectiveness of fluoride in preventing dental caries was tested comparing the frequency of caries in the children in Kingston and Newburgh. New York after Newburgh had had fluoride added to the town's drinking water. A copy of the first research report, published in 1950 can be seen here. Other community trials might test community-based interventions that provide educational programs to some communities, but not others in order to determine their effectiveness. An example might be to compare the effectiveness of a community-based educational program in Tanzania in which some villages receive the educational program, but others do not.
The Kingston-Newburgh Fluoride Trial
Prevention trials (or prophylactic trials) versus Therapeutic Trials
Clinical trials might also be distinguished based on whether they are aimed at assessing preventive interventions or evaluating new treatments for existing disease. The Physicians Health Study established that low-dose aspirin reduced the risk of myocardial infarctions (heart attacks) in males. Other trials have assessed whether exercise or low-fat diet can reduce the risk of heart disease or cancer. A study currently underway at BUSPH is testing whether peer counseling is effective in helping smokers who live in public housing quit smoking. All of these are prevention trials. In contrast, there have been many trials that have contributed to our knowledge about optimum treatment of many diseases through medication, surgery, or other medical interventions.
Phases of Trials Evaluating New Drugs
Clinical trials for new drugs are conducted in phases with different purposes that depend on the stage of development.
- Phase I trials: ClinicalTrials.gov describes phase I trials as "Initial studies to determine the metabolism and pharmacologic actions of drugs in humans, the side effects associated with increasing doses, and to gain early evidence of effectiveness; may include healthy participants and/or patients." Frequently, an experimental drug or treatment initially is tested in a small group of people (8-80) to evaluate its safety and to explore possible side effects and the doses at which they occur.
- Phase II trials: ClinicalTrials.gov describes these as "Controlled clinical studies conducted to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks." The new treatment might be tested in a somewhat larger group (80-200) to get more information about effectiveness and potential side effects at different dosages.
- Phase III trials: ClinicalTrials.gov defines these as "Expanded controlled and uncontrolled trials after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to gather additional information to evaluate the overall benefit-risk relationship of the drug and provide and adequate basis for physician labeling." These are typically conducted in larger groups (200-40,000) to formally test effectiveness and establish the frequency and severity of side effects compared to no treatment, or, compared to currently used treatments ("usual care")
- Phase IV refers to post-marketing "surveillance" to collect information regarding risks, benefits, and optimal use. This phase can be particularly important for identifying rare, but potentially devastating side effects. Example: Safety of Influenza A (H1N1) Vaccine in Post-marketing Surveillance in China