Biological Monitoring
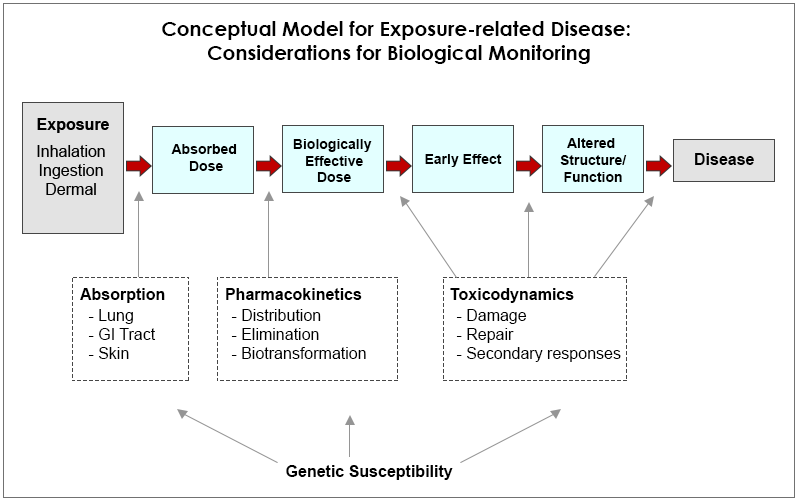
Biological monitoring involves using biomarkers to represent or estimate exposure. A biomarker is a biochemical, molecular, genetic, immunologic, or physiologic indicator of events in a biological system. As a result, biomarkers are indicators of exposure, effect, and/or susceptibility. The diagram below shows an abbreviated version of the conceptual model for exposure-related disease. This diagram highlights variables that affect the pathway of internal exposure. What is important to note is how absorption, pharmacokinetics, and toxicodynamics affect the exposure-disease pathway and subsequently will determine which biomarkers can be used to estimate exposure.
Absorption: External dose of an agent is not necessarily equivalent to the internal dose, which is affected by how the agent is absorbed into the bloodstream. Factors such as the physical and chemical properties of a given agent, the route(s) of exposure, the presence of other chemicals will affect how much of the agent is actually absorbed by the body. DEHP is not as readily absorbed through the skin when compared to ingestion.
Pharmacokinetics: The pharmacokinetics of a given agent or chemical refer to once it is absorbed, how it it distributed, metabolized/biotransformed, and eliminated. These factors will elucidate where biomarkers can be found for a given agent. Some agents are more readily excreted in urine where others are excreted in feces, which may determine which collection medium will be used to measure biomarkers. For example, dioxins tend to be lipophilic and stored in adipose tissue whereas most of absorbed lead tends to be stored in bone tissue.
Toxicodynamics: Toxicodynamics describe the mechanism of action, meaning the interactions of a toxicant with a site of action (biological target) that result in a given effect. Using the diagram below for reference, this would be the altered structure or function preceding an adverse health outcome. For example, obesogens are xenobiotic compounds that interfere with normal development and balance of lipid metabolism, which can lead to obesity. Carcinogens tend to have different toxicodynamics that result in damage to normal cells by altering DNA or the regulation/expression of DNA.
The Exposure Biology Research Group (EBRG), directed by Dr. Michael McClean, is an interdisciplinary research group in the department of Environmental Health at the Boston University School of Public Health. Their research focuses on the use of biological markers to assess environmental and occupational exposures.
Biomarker Uses and Limitations
"Biomarkers: Potential Uses and Limitations" is an article by Richard Mayeux that discusses some of the benefits and limitations to using biomarkers with respects to studying neurological diseases. Below is a summary of some of the general advantages and disadvantages of using biomarkers as indicators of exposure.
Advantages to using biomarkers: Biological monitoring allows investigators to integrate exposure via multiple pathways and can be easier to measure for long-term exposure. As a direct measure of exposure, biomarkers are free from recall bias and tend to reveal useful information on the ADME of the exposure. Biomarkers are also found close to the health outcome along the exposure-related disease pathway, so they are often relevant to the outcome of interest. Inter-individual variability with biomarkers provides important information in terms of how exposure can impact individuals differently while intra-individual variability provides some insight on how exposure changes over time. Biomarkers are also an excellent tool for evaluating the effectiveness of implemented exposure controls as well as personal protective equipment.
Disadvantages to using biomarkers: The use of biological monitoring can be expensive and require some intrusive techniques to collect data from participants. Furthermore, most biomarkers are experimental and there is limited data on "normal" populations. Other concerns are the variability in analytical techniques that can take place either within a lab or among different labs can yield different results. While inter- and intra-variability of biomarkers can provide some useful information regarding exposure, it can also pose some limitations when trying to extrapolate data collected on a sample population to the general population.
The validity of a biomarkers depends on the following variables: sensitivity, specificity, reproducibility, stability, temporal relevance, practicality. Review the tabbed activity below to learn more about these important considerations when using biomarkers.
Sensitivity
Sensitivity for a biomarker, also known as the true positive rate, is when a biomarker measurement correctly identifies subjects as being positive for the marker. Take the example of blood lead levels. Any test used to measure blood lead levels is more sensitive when it is able to correctly identify more individuals with higher blood lead levels.
Specificity
Specificity for a biomarker, also known as the true negative rate, is when a biomarker measurement correctly identifies people as negative for the health marker. Take the example of blood lead levels as a biomarker. Any test used to measure blood lead levels is more specific when it correctly identifies those with lower blood lead levels.
Reproducibility
In order for a biomarker to be valid, it must be reproducible. This means that the methods for collecting and analyzing the biomarker can yield the same results (given the same samples) over and over again as well as in different labs and/or by different investigators.
Stability
It can be difficult to find biomarkers that are stable over longer periods of time, which can present challenges particularly for prospective studies. Biomarkers can degrade over time and require certain conditions to increase their half-life.
Another aspect of stability is before collection of the biomarker takes place. Some biomarkers are unstable in temrs of how quickly they change internally after exposure has occurred. Due to the absorption, distribution, metabolism, and excretion (ADME), some agents may be excreted more readily by the body, meaning samples taken after a certain time period may underestimate exposure.
Relevance
Temporal and pathophysiological relevance should both be considered when identifying a biomarker. Being able to establish a temporal sequence where the biomarker is along the causal pathway and takes place prior to disease (e.g. increased urine creatinine is a precursor to kidney disease) further validates the dose-response effect and suggests an association between the exposure and outcome of interest.
Pathophysiological relevance means that the biomarker is indicative of the outcome of interest, or in other words, biologic plausibility. Similar to temporal sequence, pathophysiological relevance further validates the exposure-related disease pathway by establishing that an internal dose of the exposure of interest results in biophysiological changes that preclude the adverse health outcome.
Practicality
How difficult is it to obtain the biomarker measurements? A biomarker must be pratical to obtain and analyze. The more invasive the procedure to obtain biomarker data, the more impractical it is. Biomarkers that require advanced skills and unusual equipment may provide better indicators of exposure and/or outcome, but most investigators must weigh this benefit against time and resources available.
Furthermore, obtaining biomarker data must be affordable. Consider that many research studies must have a relatively large study population. Collecting expensive biomarker data from many study participants is not a practical option for many investigations.
Measuring Biomarkers
Because biomarkers consists of such a wide range of biological indicators, there are many ways to measure biomarkers as well as many different types of biomarkers. The best method will depend on the exposure of interest as well as feasibility in terms of the resources available, such as equipment and staff. Another important consideration is how invasive the procedure is in terms of collecting biomarker data from the study population. This usually involves the collection of the biomarker in a matrix (e.g. hair, skin, saliva, etc.). The following table includes examples of both invasive and non-invasive matrices through which biomarker data can be collected.
Non-invasive Matrices
In addition to being minimally invasive making subjects more likely to participate, Most of the matrices listed below require little to no advanced skills for collection.
- Breath
- Urine
- Nasal swab
- Buccal Swab
- Saliva
- Sputum
- Breast Milk
- Semen
- Hair
- Nail Clippings
- Feces
Invasive Matrices
Invasive procedures to collect biomarker data can be off-putting to potential study participants. Most of the matrices listed below require advanced skills for proper collection, which can also affect resources and time available.
- Blood lead levels
- Lung tissue
- Liver tissue
- Adipose tissue
- Bone marrow
- Bone
- Amniotic fluid
- Follicular fluid
- Bronchoalveolar lavage
- Blood vessels
Practical Application
New Exposure Biomarkers as Tools for Breast Cancer Epidemiology, Biomonitoring, and Prevention: A Systematic Approach Based on Animal Evidence is an article by Rudel et al. The article identifies biomonitoring measurement methods for chemicals that cause mammary gland tumors in animals in order to identify potential biomonitoring tools for breasts cancer studies and surveillance in humans. Review the introduction and study methods prior to answering the questions below.

