Chemical Elements: Atoms
 All matter, whether it is living or not, is composed of chemical elements; these are fundamental chemicals in the sense that they are what they are - they can't be changed into another element. Each element is distinguished by the number of protons
All matter, whether it is living or not, is composed of chemical elements; these are fundamental chemicals in the sense that they are what they are - they can't be changed into another element. Each element is distinguished by the number of protons , neutrons
, neutrons , and electrons
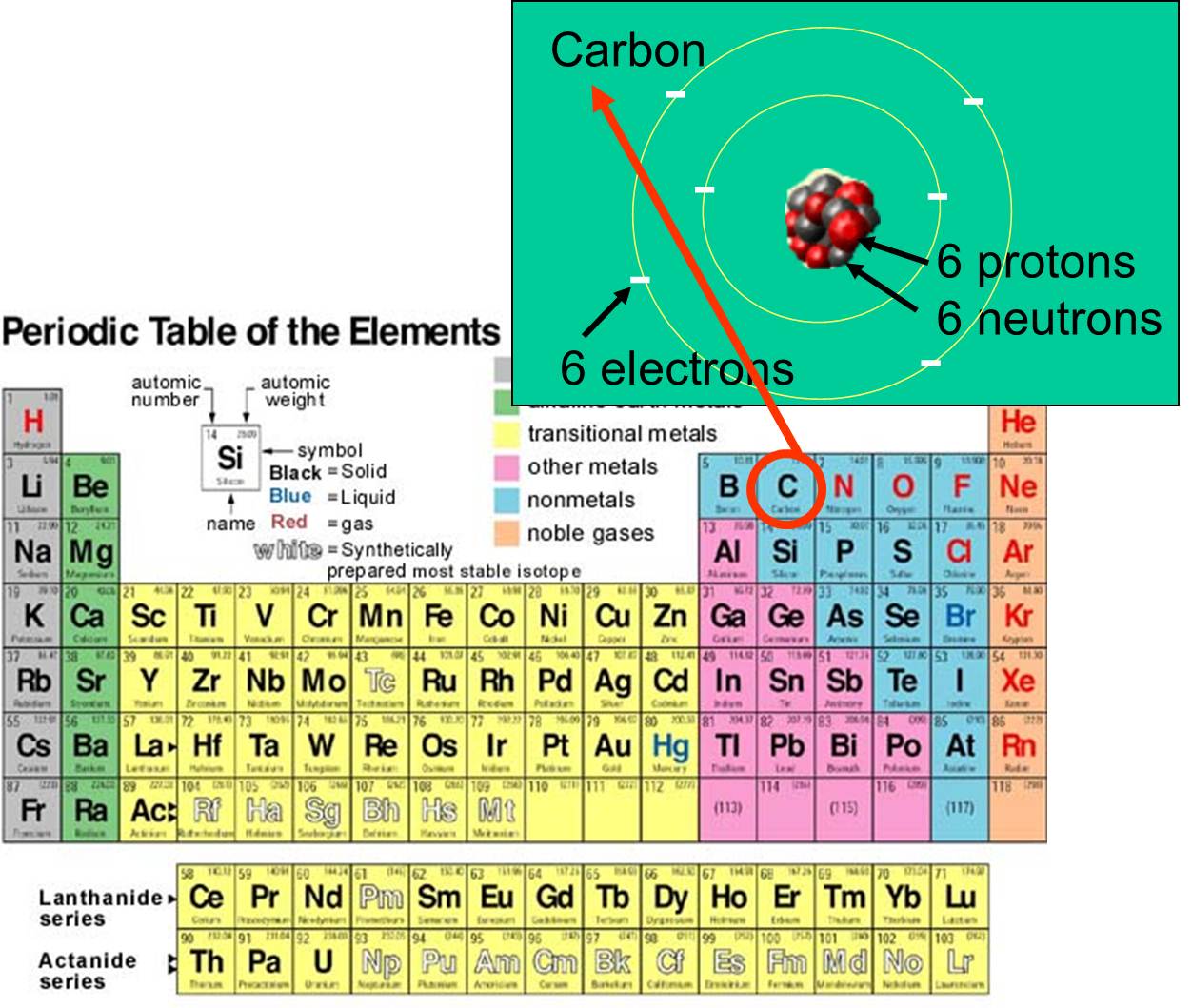
, and electrons that it possess. For example, carbon's atomic number is 6, and has an atomic mass of about 12, because it has 6 positively charged protons and 6 non-charged neutrons. The 6 charged electrons contribute very little to the atomic mass. There are 92 naturally-occurring elements on earth. The array of elements and their subatomic structure are summarized by the periodic table of the elements, shown to the right.
that it possess. For example, carbon's atomic number is 6, and has an atomic mass of about 12, because it has 6 positively charged protons and 6 non-charged neutrons. The 6 charged electrons contribute very little to the atomic mass. There are 92 naturally-occurring elements on earth. The array of elements and their subatomic structure are summarized by the periodic table of the elements, shown to the right.
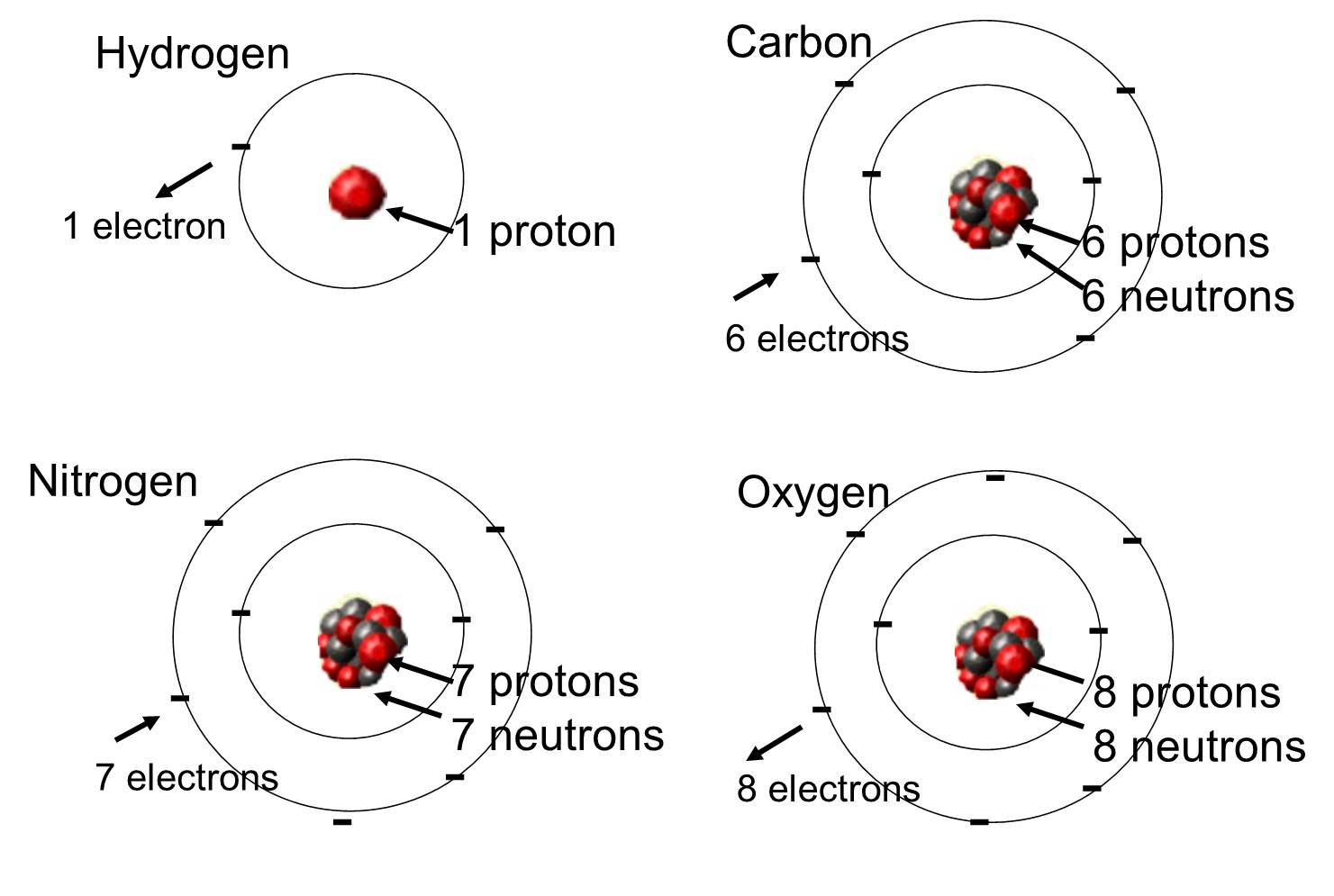
In living organisms the most abundant elements are carbon, hydrogen, and oxygen. These three elements along with nitrogen, phosphorus, and a handful of other elements account for the vast majority of living matter. An atom is one single unit of a chemical element. Some of these elements that are abundant in organic molecules are shown below.
Molecules and Chemical Bonds
 Atoms can combine with other atoms by forming chemical bonds.
Atoms can combine with other atoms by forming chemical bonds.
Covalent Bonds
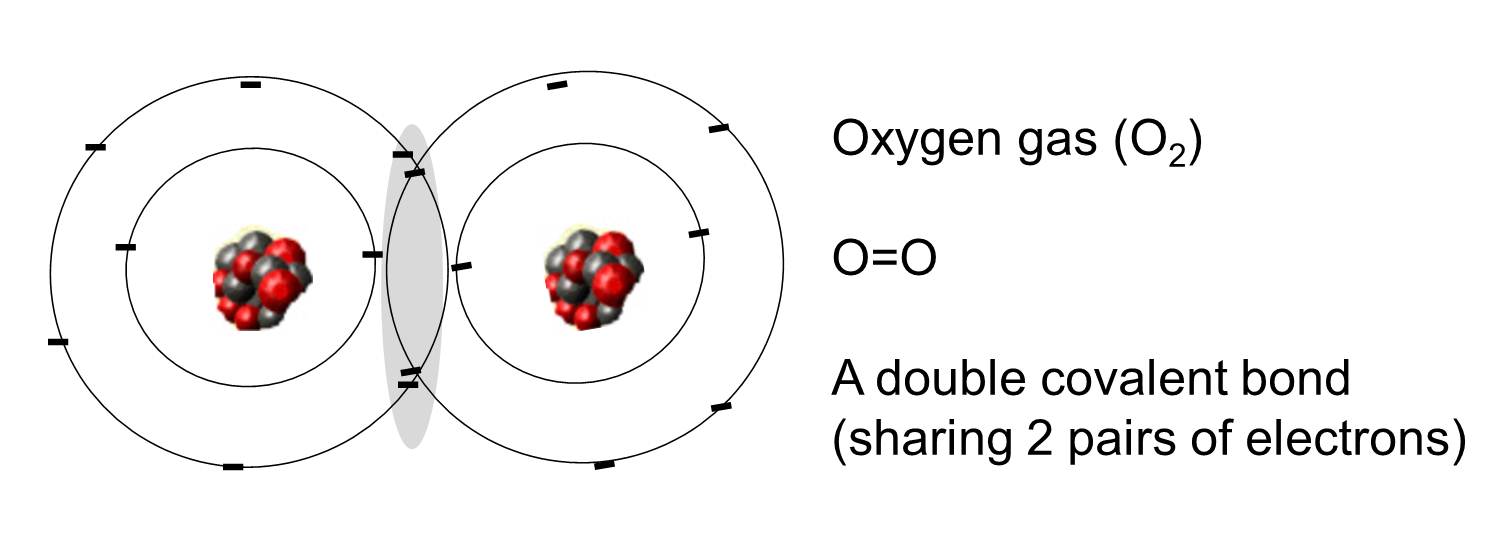
A covalent bond is one in which one or more pairs of electrons are shared by two atoms. The illustration to the right shows two atoms of oxygen that are covalently bonded by the sharing of two pairs of electrons as illustrated in the shaded area.
The figure below shows a series of molecules formed by covalent binding. Mouse over each molecule to see a brief description.
, 
Water is a Polar Molecule
Note also that the sharing of electrons is not always equal. For example, in a water molecule, the negatively charged electrons spend more time in the vicinity of the heavier oxygen atom.
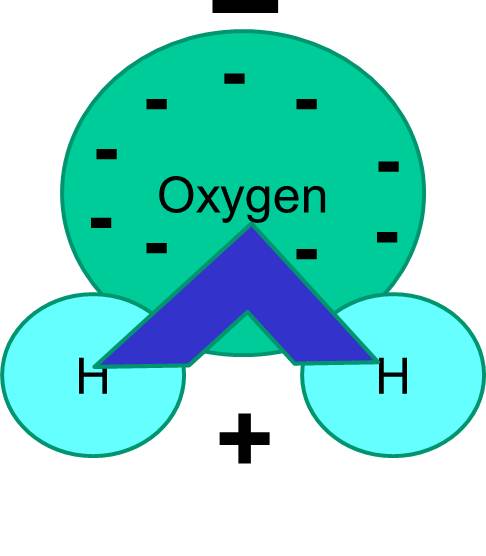
The net result is that the water molecule has one end that is more negative relative to the other end. Water is therefore a "polar" molecule. We will see that this polarity has important implications for many biological phenomena including cell structure. You may have heard the expression "like dissolves like." What this means is that polar molecules dissolve well in polar fluids like water. Sugars (e.g., glucose) and salts are polar molecules, and they dissolve in water, because the positive and negative parts of the two types of molecules can distribute themselves comfortably among one another.
Ionic Bonds
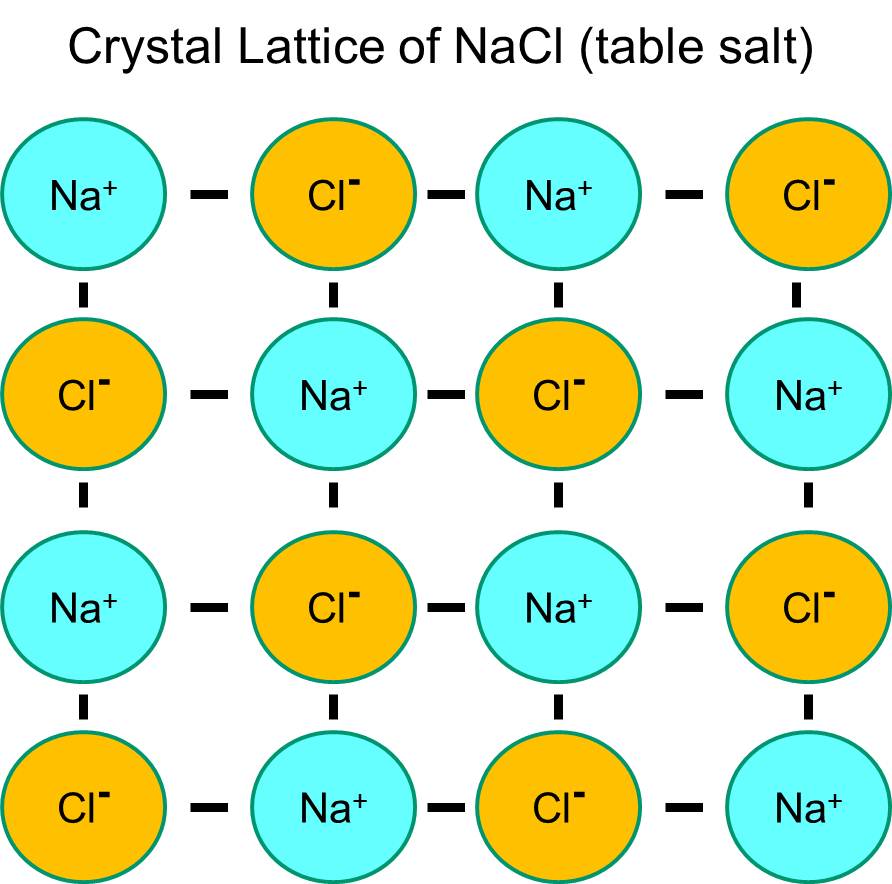
Sodium has a single electron in its outermost orbital shell, and it is thermodynamically more stable if it gives up this electron. This loss of a negative electron results in a positively charged sodium ion, abbreviated Na+. Chlorine, on the other hand, has seven electrons in its outermost orbital shell, and it is more thermodynamically stable if it acquires an extra electron to complete the outer orbital shell. This results in a negatively charged chloride ion, abbreviated Na+. The positively charged sodium ions and the negatively charged chloride ions attract each other and result in the formation of an ionic bond. In the absence of water, sodium and chloride form a crystal lattice because of the attraction of negative and positive ions.
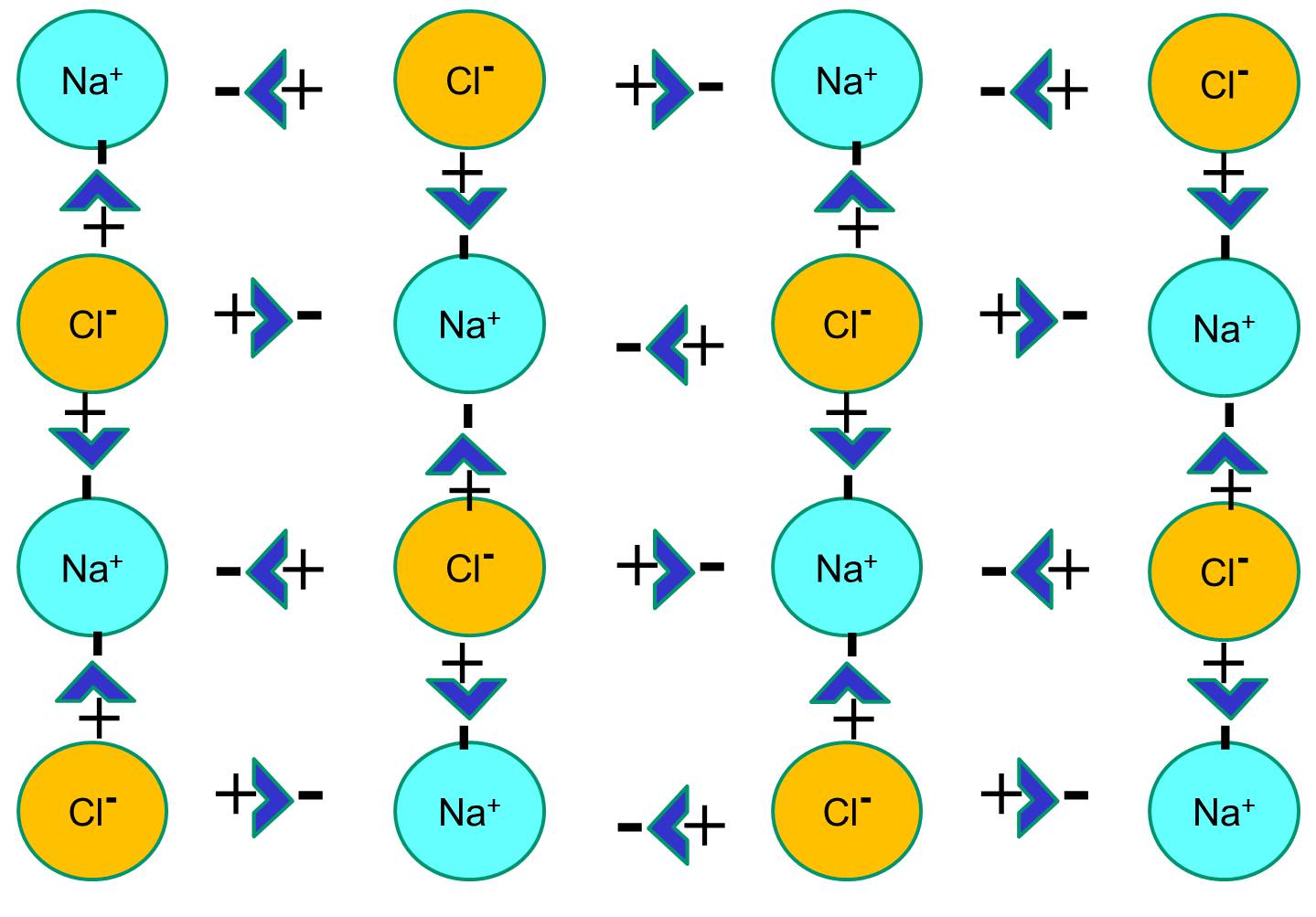
However, if sodium chloride crystals are placed in water, the polar water molecules will "hydrate" the sodium and chloride atoms because the water molecules are polar. In the illustration below the darker blue V-shaped figures represent water molecules, which are polar. The positive ends of the water molecules are attracted to the negatively charged chloride ions, while the negative pole of the water molecule is attracted to the positive sodium ions. As a result, the ions are hydrated and the crystal lattice dissolves into the aqueous solution. This is exactly what happens when you add crystalline table salt to a glass of water.
The video below provides an animated explanation of how salts like NaCl dissolve in water.