What Can be Done? (Evidence Based Solutions)
Currently, there are no known vaccines or treatments for EEE or WNV. Supportive treatments are available to relieve some pain and certain symptoms. Therefore, evidence based control measures are crucial to preventing the development of cases. There are multiple control methods available that vary both in terms of time period of implementation and program type. In addition to having control methods, surveillance is a measure that can be taken by the state or locally to measure and predict levels of the virus.
Surveillance
Public Health surveillance begins at the local level with diagnosing and confirming cases of WNV and EEE. Each state has laws that require providers and laboratories to report confirmed cases of specific diseases, including WNV and EEE, to the State Health Departments. Once the data has been reported, it helps inform the State Health Departments about which interventions, if any, are needed. The CDC then encourages that states pass on their data, so that the CDC can have a view of the prevalence of WNV and EEE nationwide. The CDC uses the National Notifiable Disease Surveillance System (NNDSS) to help monitor the occurrence and spread of WNV and EEE. The NNDSS is used for a variety of purposes including to identify disease trends, maintain national standards, and to work with states to help implement prevention and control measures.
Passive and Active surveillance- The passive surveillance system in the US is the NNDSS, as mentioned previously. The NNDSS regularly monitors and keeps track of various diseases nationally. Active surveillance often involves short-term initiatives such as communicating current surveillance information, promoting disease prevention strategies, reviewing specimen submission procedures, and highlighting the need for testing patients that have the signs and symptoms of either EEE or WNV. This can take place if there is a high risk of disease.
Mosquitoes- One method of mosquito surveillance is trapping. Gravid mosquitoes are trapped throughout the arbovirus season. These mosquitoes are then counted and sorted by species where they are tested for WNV and EEE in "mosquito pools".
Fixed and long-term trap sites provide several benefits. They are useful for predicting mosquito population levels and virus prevalence. They are also useful for estimating the relative risk of human infection from EEE and WNV. In addition to trapping mosquitoes, mosquito larvae from select sites in late fall and early spring to determine end season and preseason larval abundance in addition to checking up frequently during arbovirus.
Animal- If horses or other domestic animals have several neurological diseases and it is suspected to be caused by EEE or WNV, they are tested. Veterinarians, USDA, and other sources will collaborate together to identify and report suspect animal cases. Blood and/or tissue samples from animals are also tested when appropriate. Horses and other animals can be immunized against infection with WNV and EEE and this is considered to be the primary means of preventing infection in animals.
Human- Testing for either EEE of WNV consists of a preliminary screening test (enzyme immunoassay), followed by confirmatory testing by plaque reduction neutralization in specimens or PCR. It is important to note that the antibodies for WNV can last for months; therefore a positive test does not mean there is a current infection.
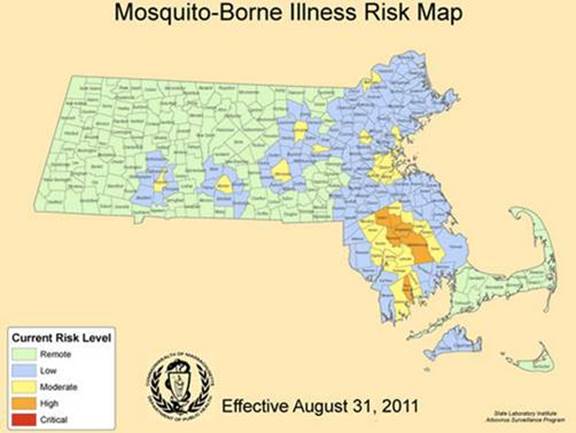
Figure 7: A map of Massachusetts that shows the risk levels of the counties. This is a map that would be used to the determine which areas need immediate intervention. (http://www.mysouthborough.com/2012/08/17/threat-level-for-eee-in-southborough-raised-to-moderate/)
Diagnostic Methods and Screening
Diagnosing and reporting WNV and EEE relies upon two tests that use nucleic acids as a sign that a person has been infected with either virus. It is important that diagnostic methods are accurate in order to better track where infected mosquitoes inhabit and if those areas then need an intervention strategy to be applied.
Nucleic Acid Test- NAT screening for WNV was seen as a potential measure to prevent viral spread by transfusion of blood and components and from donation of organs and tissues. Testing plasma specimens to screen organ donors when the specimen is obtained while the donor's heart is still beating, and in testing blood specimens to screen cadaveric (non-heart beating) donors.
RNA detection: Procleix®WNV Assay, developed by Gen-Probe Inc. and marketed by Chiron Corporation, for the detection of WNV RNA in the plasma specimens from individual human donors including volunteer donors of whole blood and blood components and other living donors. This PCR test can also be use to test for WNV RNA fragments but this method does have a low sensitivity rate
One of the more preferred methods of diagnosis to see if it has infiltrated the central nervous system. This involves identifying IgM immunoglobulin antibodies in the cerebrospinal fluid. During the 8th day of illness most people will have detectable levels of IgM and will remain detectable for a couple of months. IgG antibodies can be detected 3 weeks after the infection.
Universal screening for WNV is not a cost-effective intervention, even in areas with high rates of natural transmission of WNV. In areas with high rates of transmission of WNV, seasonal, targeted screening of donations for immunocompromised individuals may be cost-effective, subject to pricing of screening assays.
