Authors:
Caroline Yang, Boston University
Sarah Esselborn, Boston University
Shannon Linderman, Boston University
Valorie Richards, Boston University
Kimberly Barrett, Boston University
RK: I think it's nice that you linked out to alot of external resources, but I would have liked to have seen you weave relevant pieces of information throughout your content. The intended audience is your fellow public health practitioners and I think there the style of the module is more geared towards a general audience….not necessarily professionals in the field.
I would have also liked the different sections to flow a little better into each other, and it would have been nice to have a conclusion page to wrap up the module at the end.
Contrary to what their names suggest, West Nile Virus (WNV) and Eastern Equine Encephalitis (EEE) are not exclusively constrained to a specific geographic area, but are pathological viruses that are endemic to many regions of the world. Both are neuro-invasive viruses with similar structure and transmission mechanisms that vary slightly in symptom presentation and areas where prevalent. Therefore, it is important to understand the differences between these viruses, while appreciating their similarities when considering public health control measures that could be used to protect large populations from these viruses and their potentially devastating health effects.

Figure 1: A mosquito acting as a possible vector for WNV. (http://science.howstuffworks.com/zoology/insects-arachnids/mosquito.htm)
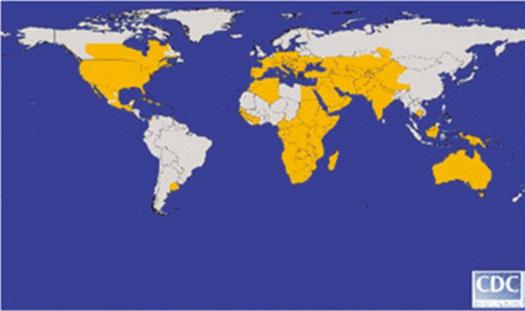
Figure 2: Global distribution of WNV in 2006. (http://cid.oxfordjournals.org/content/45/8/1039.long)
After successful completion of this section, the student or entry-level professional will be able to:
1. Define arbovirus and describe the most common reservoir and vector for WNV and EEE
2. Describe the transmission cycle of WNV and EEE
3. Understand factors that contribute to risk of infection
4. Describe the disease impact on humans
5. Discuss evidence-based strategies for prevention of WNV and EEE
6. Discuss the problem with the use of pesticides
7. Provide examples of interventions at each level
8. Differentiate between the possible interventions that could be done at different levels (resident vs. state)
9. Identify the problem with aerial sprays
10. Provide examples of surveillance of WNV and EEE
Eastern Equine Encephalitis (EEE) and West Nile Virus (WNV) are single stranded RNA arboviruses.
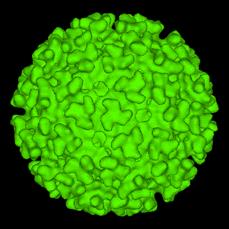
Figure 3: Computer-generated model of EEE surface based on cryo-electron microscopy.
(http://www.cdc.gov/ncidod/dvbid/arbor/images/alphavir.gif)
For more information on the spread of West Nile Virus into the western hemisphere, see: http://www.nejm.org/doi/full/10.1056/NEJMp048261, and
http://www.nejm.org/doi/full/10.1056/NEJMp1210537Mosquitoes can become infected with EEE or WNV from feeding on infected birds that are reservoirs for both viruses. Infected mosquitoes transmit the disease directly to humans through biting.
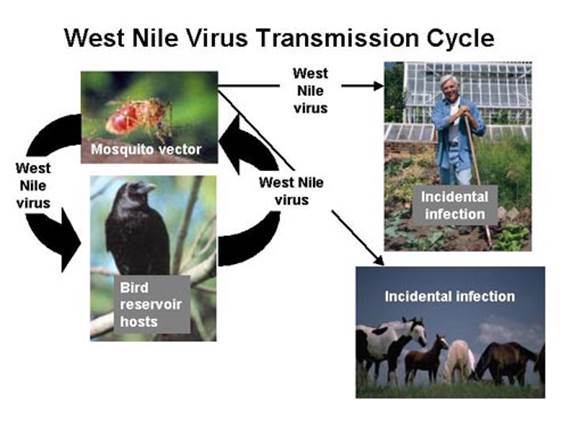
Figure 4: The Transmission Cycle of West Nile Virus and Eastern Equine Encephalitis
(http://nationalatlas.gov/articles/biology/a_wnv.html).
Through an enzootic cycle involving mosquitoes and birds, the viruses are able to survive in nature, which facilitates ongoing transmission from infected birds to uninfected mosquitoes, which can then be transmitted to humans (Figure 4).
RK:I think this section would have fit better at the end of all the human infection-related subsections. It talks about a lot of rare events, but there is no numerical evidence (incidence or prevalence) to back up some of the facts.
Several rare reports describe the vertical transmission of both viruses from pregnant or breastfeeding women to their babies. The possibility for transmission between humans through blood transfusions or organ transplants also exists.
Mosquitos have been documented to occasionally transmit these infections to horses and other animals. Although there has never been documented transmission from birds to people, dead birds have the potential to transmit the viruses to humans if handled with bare hands. Therefore, special precautions need to be taken when handling dead animals, especially birds that may be infected with WNV or EEE.
For additional information on transmission of WNV to horses:
http://www.sciencedirect.com/science/article/pii/S0378113513004124
Upon transmission, viral replication begins at the bite site. The virus then spreads to the lymph nodes and blood stream. The infection can also spread to the neurons of the central nervous system (especially in dark matter), which may increase blood-brain barrier permeability and cause encephalitis or meningitis, some of the most serious symptoms associated with these viruses.
The following video shows the sequence of how a virus enters a host cell to replicate its DNA. This is a general sequence of events, not specific to WNV or EEE, but is an excellent model for how those viruses replicate as well.
Simple viral replication video (34 seconds):
Approximately 80% of infected individuals will experience relatively mild symptoms or be asymptomatic. However, development of mild and severe symptoms may occur 3 to 14 days after a bite from an infected mosquito. Table 1 summarizes the symptoms of WNV and EEE, and table 2 summarizes the incidence of symptom severity:
Table 1: Possible mild and severe symptoms of WNV and EEE.
|
|
West Nile Virus |
Eastern Equine Encephalitis |
|---|---|---|
|
Mild Symptoms |
|
|
|
Severe Symptoms |
|
|
For further reading on the symptoms of WNV, read:
http://www.nejm.org/doi/full/10.1056/NEJM200106143442401#t=article
For further reading on the symptoms of EEE, read:
http://www.nejm.org/doi/full/10.1056/NEJMoa1212628#t=article
Table 2: Incidence of West Nile Virus by Degree of Severity
|
West Nile Virus |
|
|---|---|
|
No Symptoms |
70-80% |
|
Mild Symptoms |
20% |
|
Severe Symptoms |
1% |
|
Deaths |
10% |
EEE shares a similar distribution of disease severity, with most infected people showing no apparent symptoms. However, death occurs in approximately 33% of patients. 35-50% of those who survive the disease will have permanent neurological disorder.
For further reading on the infection and pathogenesis of WNV: http://www.mdpi.com/1999-4915/5/11/2856


RK: "Who" is at risk for infection makes me think that this page will be about human risk factors...but then there is a subsection about environmental conditions which is a little confusing.
Since EEE and WNV are arboviruses usually spread by mosquitos, both share similar disease determinants associated with the environment, personal behaviors, and physical characteristics of a person.
The main environmental determinant for EEE and WNV is the amount of time spent in a region inhabited by infected mosquitoes. High-risk regions for WNV in the U.S. include Southern and Midwestern States (Figure 5). Freshwater hardwood swamps in Atlantic or Gulf Coast states are considered high-risk areas for EEE. States most affected by EEE include Florida, Georgia, Massachusetts and New Jersey (Figure 6). Other environmental factors that influence the likelihood of virus transmission include the presence of ideal mosquito breeding conditions like standing water or dense vegetation. Economic factors may also dictate the availability of community mosquito abatement programs that can significantly lower infection rates in specific areas.
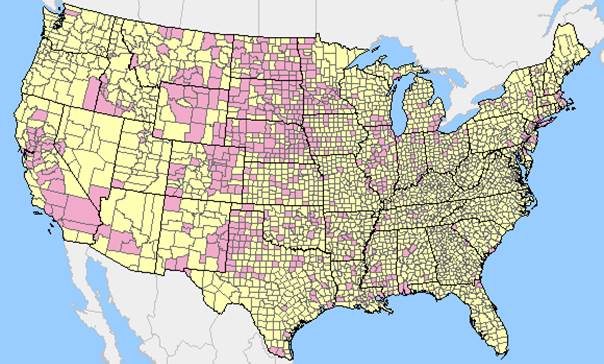
Figure 5: Positive WNV test results (pink) compared to U.S. counties with no positive WNV results (yellow) reported for the 2012 surveillance cycle.
(http://diseasemaps.usgs.gov/wnv_us_human.html)
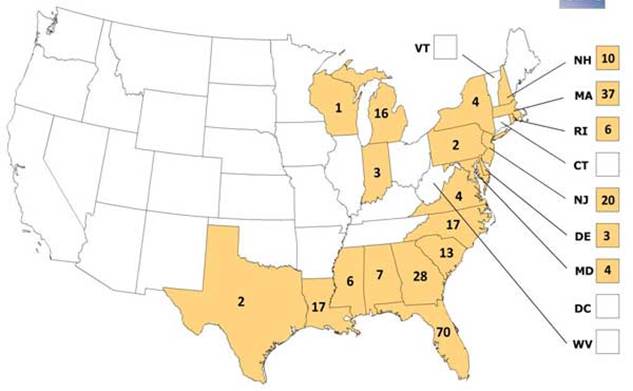
Figure 6: Number of Observed Cases of EEE by U.S. State from 1964-2010
(http://www.cdc.gov/easternequineencephalitis/images/eee_state_map_600px.jpg)
Personal behaviors that increase the likelihood of mosquito exposure will increase the likelihood of disease development. The amount of time one spends outside and compliance with personal protective behaviors to limit acquisition of mosquito bites directly affect one's risk of disease. Also, actions to eliminate mosquito populations and insect breeding grounds on personal property are key to lowering personal risk of disease. Examples of recommended preventative actions include wearing long-sleeved clothing outside at times of peak mosquito activity and eliminating standing water in flower pots and roof gutters.
However, studies indicate that many individuals fail to observe recommended personal protective behaviors, despite having knowledge of the disease these behaviors protect against and having received information from a local or state health department. Some reports suggest that age is the most reliable predictor of failure to comply with personal protective behaviors.
For further reading on studies that analyze the uptake of personal protective behaviors for WNV:
http://www.ncbi.nlm.nih.gov/pubmed/18457290
Study on uptake of personal protective behaviors for WNV:
http://www.ncbi.nlm.nih.gov/pubmed/18457290
Physical characteristics like age and immune system health also play a role in the risk of disease transmission. Individuals below 15 years of age or above age 50 are most likely to become infected. However, severe symptoms, such as encephalitis and meningitis, are most likely to occur in individuals over the age of 50. Age may also influence practice of personal protective behaviors that significantly affect disease transmission.
Some investigators cite that children are greater risk for contracting WNV due to their typical higher levels of outdoor exposure, failure to avoid going outside and wearing long-sleeves compared to older populations. Other studies mention that older individuals (above 51 years of age) are less likely modify their daily actions such as wearing mosquito repellent containing DEET when outdoors and dawn or dusk.
Immunocompromised individuals such as HIV+ individuals or patients that have undergone recent chemotherapy or organ transplants are also at higher risk of infection. Serological surveys also indicate that a low prevalence of antibodies to WNV can be associated with areas of significant WNV transmission.
Study on increased WNV risk for children due to failure to observe personal protective behaviors:
http://www.ncbi.nlm.nih.gov/pubmed/17518307
Study that observes older adult's failure to wear mosquito repellent:
http://www.ncbi.nlm.nih.gov/pubmed/15850029
Serological studies for areas of intense WNV transmission:
http://www.ncbi.nlm.nih.gov/pubmed/17518307

Currently, there are no known vaccines or treatments for EEE or WNV. Supportive treatments are available to relieve some pain and certain symptoms. Therefore, evidence based control measures are crucial to preventing the development of cases. There are multiple control methods available that vary both in terms of time period of implementation and program type. In addition to having control methods, surveillance is a measure that can be taken by the state or locally to measure and predict levels of the virus.
Public Health surveillance begins at the local level with diagnosing and confirming cases of WNV and EEE. Each state has laws that require providers and laboratories to report confirmed cases of specific diseases, including WNV and EEE, to the State Health Departments. Once the data has been reported, it helps inform the State Health Departments about which interventions, if any, are needed. The CDC then encourages that states pass on their data, so that the CDC can have a view of the prevalence of WNV and EEE nationwide. The CDC uses the National Notifiable Disease Surveillance System (NNDSS) to help monitor the occurrence and spread of WNV and EEE. The NNDSS is used for a variety of purposes including to identify disease trends, maintain national standards, and to work with states to help implement prevention and control measures.
Passive and Active surveillance- The passive surveillance system in the US is the NNDSS, as mentioned previously. The NNDSS regularly monitors and keeps track of various diseases nationally. Active surveillance often involves short-term initiatives such as communicating current surveillance information, promoting disease prevention strategies, reviewing specimen submission procedures, and highlighting the need for testing patients that have the signs and symptoms of either EEE or WNV. This can take place if there is a high risk of disease.
Mosquitoes- One method of mosquito surveillance is trapping. Gravid mosquitoes are trapped throughout the arbovirus season. These mosquitoes are then counted and sorted by species where they are tested for WNV and EEE in "mosquito pools".
Fixed and long-term trap sites provide several benefits. They are useful for predicting mosquito population levels and virus prevalence. They are also useful for estimating the relative risk of human infection from EEE and WNV. In addition to trapping mosquitoes, mosquito larvae from select sites in late fall and early spring to determine end season and preseason larval abundance in addition to checking up frequently during arbovirus.
Animal- If horses or other domestic animals have several neurological diseases and it is suspected to be caused by EEE or WNV, they are tested. Veterinarians, USDA, and other sources will collaborate together to identify and report suspect animal cases. Blood and/or tissue samples from animals are also tested when appropriate. Horses and other animals can be immunized against infection with WNV and EEE and this is considered to be the primary means of preventing infection in animals.
Human- Testing for either EEE of WNV consists of a preliminary screening test (enzyme immunoassay), followed by confirmatory testing by plaque reduction neutralization in specimens or PCR. It is important to note that the antibodies for WNV can last for months; therefore a positive test does not mean there is a current infection.
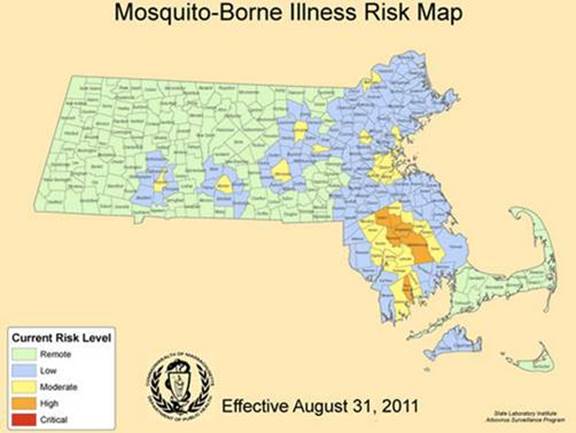
Figure 7: A map of Massachusetts that shows the risk levels of the counties. This is a map that would be used to the determine which areas need immediate intervention. (http://www.mysouthborough.com/2012/08/17/threat-level-for-eee-in-southborough-raised-to-moderate/)
Diagnosing and reporting WNV and EEE relies upon two tests that use nucleic acids as a sign that a person has been infected with either virus. It is important that diagnostic methods are accurate in order to better track where infected mosquitoes inhabit and if those areas then need an intervention strategy to be applied.
Nucleic Acid Test- NAT screening for WNV was seen as a potential measure to prevent viral spread by transfusion of blood and components and from donation of organs and tissues. Testing plasma specimens to screen organ donors when the specimen is obtained while the donor's heart is still beating, and in testing blood specimens to screen cadaveric (non-heart beating) donors.
RNA detection: Procleix®WNV Assay, developed by Gen-Probe Inc. and marketed by Chiron Corporation, for the detection of WNV RNA in the plasma specimens from individual human donors including volunteer donors of whole blood and blood components and other living donors. This PCR test can also be use to test for WNV RNA fragments but this method does have a low sensitivity rate
One of the more preferred methods of diagnosis to see if it has infiltrated the central nervous system. This involves identifying IgM immunoglobulin antibodies in the cerebrospinal fluid. During the 8th day of illness most people will have detectable levels of IgM and will remain detectable for a couple of months. IgG antibodies can be detected 3 weeks after the infection.
Universal screening for WNV is not a cost-effective intervention, even in areas with high rates of natural transmission of WNV. In areas with high rates of transmission of WNV, seasonal, targeted screening of donations for immunocompromised individuals may be cost-effective, subject to pricing of screening assays.
RK: How would a public health practioner determine which control option (reactive vs proactive) is better?
Control measures can be implemented proactively or as a reaction to confirmed cases of disease.
Proactive control involves prevention strategies and has been shown to be more effective than reactive methods.
Reactive control involves intervening during or after mosquito season has already started and there are already confirmed cases.
· Proactive example: Integrated Pest management
· Reactive examples: Passive case surveillance followed by adulticide application
Integrated Pest Management involves monitoring pest populations while preventative measures are in place, followed by the use of control measures when pest populations or environmental conditions exceed pre-set thresholds. Control methods usually consist of environmentally sensitive measures that rely on interruption of pest life cycles and their environmental interactions. This method of management typically involves a combination of the following measures: source reduction and surveillance programs. Source Reduction Efforts eliminate larval habitats or render them uninhabitable. These efforts are a joint effort between the state, towns, and individuals to eliminate any possible habitats for mosquito larvae. Surveillance programs track disease harbored by wild birds & sentinel chicken flocks, adult and larval mosquitoes, or biting counts. Such programs can follow-up on public complaints or reports by the public. Seasonal records are recorded and published.
Interventions occur at the state, local, and individual level. The following text highlights the main methods used at each level.
There are several different types of intervention strategies against WNV and EEE at the state level.
The first type includes biological control methods. These methods are environmentally safe ways of reducing or mitigating infected mosquito populations through the use of their natural predators like Mosquito fish (Gambusia affinis and G. holbrooki), predacious mosquitos (Toxorhynchites) or entomopathic fungi (Liginex). For example the Gambusia affinis, as seen in Figure 8, will eat the larvae of mosquitoes.

Figure 9: Figure 9: Gambusia affinis, an example of a biological control, will eat the larvae of mosquitoes. http://ucanr.edu/sites/coast/Mosquito_Fish/
However, a drawback is that these measures can negatively affect ecosystems through indiscriminate killing of other populations like tadpoles, zooplankton, and aquatic insects.
Another action the state can take is to employ Open Marsh Water Management. This program is a method that controls mosquitoes through the excavation of shallow pools and ditches, not connected to tidal channel, in mosquito-breeding areas. This creates pools with a higher water level that results in a habitat for fish and other wildlife that can prey upon mosquito larvae and pupae.
Another intervention by the state can include mosquito traps. These devices use compressed CO2, propane, or octenol to capture adult mosquitos. However, these devices are very expensive and a better strategy would be to target mosquitoes earlier in the life cycle.
|
Fun Fact: High voltage electric insect traps (i.e. Bug Zappers) can also kill mosquitoes. |
A final type of intervention involves the use of pesticides. Pesticides are substances used for killing insects or other organisms harmful to animals or cultivated plants. There are two main types: larvicides and adulticides. As the names suggest, they each attack mosquitoes in a different phase of their life cycles. Larvicides are proactive measures that kill large numbers of immature mosquitoes, which can prevent a large viral outbreak. On the other hand, adulticides target adult mosquitoes and prevent large swarms from emerging. Adulticides can be administered in a large or small droplet forms. The small droplets are more effective at coming into contact with and ultimately killing, mosquitoes. Adulticides consist of large droplets that are more likely to settle on the ground or vegetation rather than contacting mosquitoes. Not only does this method kill fewer mosquitoes, but it can also have undesirable effects on non-targeted organisms.
During seasons with increased presence of WNV and EEE, the state can conduct aerial adulticide sprays throughout high-risk areas (Figure 9). The sprays consist of the chemicals sumithrin and piperonyl butoxide (PBO), which can cause minor health problems for people if necessary precautions are not taken (e.g. staying indoors during application and closing windows). The sprays can also affect non-target insects, leading to widespread adulticide. Therefore, it would be best if the state reserved aerial spraying for emergencies. Although aerial sprays done at a state level can reduce risk, it does not eliminate the viruses and can cause unwanted environmental effects. Additionally, widespread aerial spraying has the risk of conveying a false sense of security to residents.
Timed minimal application rates and delivery mechanisms approved by trained public health professionals can minimize adverse effects of pesticides. It is important that the sprayings of pesticides do not harm non-targeted organisms. Low rates of application can mitigate any hazard to other animals, such as honeybees, ladybug, and butterflies. It is also important that human exposure to pesticides is decreased through night applications and public notification prior to spraying.

Figure 10: Truck spraying town streets with pesticides. The spraying is done at night to reduce direct exposure to residents (http://www.meepi.org/wnv/overkillma.htm)
In order to prevent high exposure from mosquitoes, towns should advise their residents to limit outdoor activities, such as gardening, during high risk times and to take measures to protect themselves. Times when exposures to mosquitoes are highest are during sunrise, sunset, and early evening, especially during the months of July-September. Towns and schools should consider scheduling events indoors when mosquitoes are most active, such as during early evenings and the summer. During an outbreak, towns should intensify the focus on personal prevention methods, such as the use of repellent. Local officials should keep each other and the public informed and up-to-date with the identified cases during the high-risk season.
Towns and residents can also take part in spraying pesticides. One way is through misting systems, which are outdoor residential systems that use nozzles mounted around buildings to spray pesticides. Common pesticides like pyrethrins and permethrin are only active in the environment for a short time period, so human exposure can be reduced by avoidance of misting areas during designated times. However, insufficient data exists to confirm the effects of misting systems on mosquito control.
For additional information on the role of pesticides in integrated mosquito management, see: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2631680/pdf/11266290.pdf

Figure 11: Quick summary of basic actions that people can take to protect themselves from mosquitoes infected with WNV and EEE
(http://www.roxbydowns.com/breaking-news/mosquito-control)
The best prevention methods against infections from WNV and EEE are simple actions that all individuals can carry out. First, in order to reduce the number of mosquito bites acquired while outside, residents should wear clothing, such as long pants and long sleeves that covers open skin. People should also cover gaps in baby carriages with netting while outside (Figure 12). It would also be prudent for residents to limit time spent outdoors during times when mosquitoes are most active. Finally, people should wear insect repellent while outdoors.

Figure 12: Mosquito net to protect infants while outside.
(http://www.equipoutdoors.co.nz/contents/en-us/d617_mosquito_nets.html)
Repellents are chemicals applied to an individual's clothing or exposed skin to discourage mosquito bites and decrease the likelihood of disease transmission. There are a variety of commercial products that contain effective chemicals for warding off mosquitoes. The four most effective repellent active ingredients are: DEET, Picaridin, IR3535, and oil of lemon eucalyptus. Products containing DEET (N, N-diethyl Metatoluamide) are the most common and have the largest number of hourly protection (~8-12 hours) per application. However, a drawback to DEET is that it is toxic at extremely high levels for freshwater fish and insects. Repellents that include the active ingredient Picaridin are moderately toxic to fish. Finally, repellents containing oil of lemon eucalyptus are not recommended for use on children under the age of three years. While insect repellents are an excellent way to protect oneself from mosquitoes and thus potential disease transmission, exclusive use of repellents is not enough to prevent WNV or EEE transmission.
|
Fun Fact: Despite the existence of dozens of brands, the most common active ingredient in insect repellents is DEET. |
In addition to strategies that protect an individual from mosquito bites, residents can modify personal property to reduce risk of WNV and EEE transmission. The best action that residents can take is to reduce areas of standing water on their property. Mosquitoes lay eggs in standing water and it would drastically decrease the mosquito population to remove areas for larval development. Examples of action to eliminate larval habitats or render them uninhabitable include: emptying and changing water in bird baths, pools, fountains, buckets, gutters, tires etc. once a week to destroy potential habitats; keeping swimming pool water treated. Homeowners can also fill in ditches and holes in trees, which can also become possible habitats for mosquitoes (Figure 13).
In addition to limiting larval habitats, residents can protect themselves by covering gaps in walls, doors, and windows to prevent mosquitoes from entering the house. Residents should also make sure screens in windows and doors are bug-tight. An additional layer of protection is to cover cribs and beds with netting. Replacing outdoor lights with yellow "bug" lights tend to attract fewer mosquitoes as well.


http://www.mass.gov/eohhs/docs/dph/cdc/arbovirus/eee-expert-panel-raynham-town-meeting.pdf
http://www.journals.elsevier.com/biological-control/
http://www.ct.gov/mosquito/cwp/view.asp?a=3486&q=415084
http://www.cdc.gov/nceh/ehs/Docs/JEH/2013/april-wnv.pdf
http://www.veterinaryresearch.org/content/43/1/16
http://online.liebertpub.com/doi/pdf/10.1089/vbz.2010.0040
http://cfpub.epa.gov/oppref/insect/#searchform
http://npic.orst.edu/factsheets/PicaridinGen.html
https://public.health.oregon.gov/diseasesconditions/diseasesaz/westnilevirus/pages/wnvprevent.aspx
https://public.health.oregon.gov/DiseasesConditions/DiseasesAZ/WestNileVirus/Documents/multpeng.pdf
Article on some prevention methods against WNV: http://www.cdc.gov/nceh/ehs/Docs/JEH/2013/april-wnv.pdf
http://www2.epa.gov/mosquitocontrol/mosquito-misting-systems
http://www.ncbi.nlm.nih.gov/pubmed/22703681
http://www.cdc.gov/westnile/resources/pdfs/wnvGuidelines.pdf
http://www.mass.gov/eohhs/docs/dph/cdc/arbovirus/arbovirus-surveillance-plan.pdf
http://www.mayoclinic.com/health/west-nile-virus/DS00438/DSECTION=risk%2Dfactors
http://health.nytimes.com/health/guides/disease/west-nile-virus/background.html
http://www.cdc.gov/EasternEquineEncephalitis/tech/factSheet.html
http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/eastern-equine-encephalitis
http://www.cdc.gov/EasternEquineEncephalitis/tech/epi.html
Geographic Distribution of WNV cases:
http://www.cdc.gov/westnile/statsMaps/
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4300B2_02.htm
http://cid.oxfordjournals.org/content/43/4/490.full
http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/88680
http://www.mass.gov/eohhs/docs/dph/cdc/arbovirus/arbovirus-surveillance-plan.pdf
http://www.mysouthborough.com/wp-content/uploads/2011/09/20110906-cmmcp-risk-map-sm.jpg
http://wwwn.cdc.gov/nndss/
http://www.cdc.gov/vaccines/pubs/surv-manual/chpt19-enhancing-surv.html