Functions of Proteins
Oxygen Transport
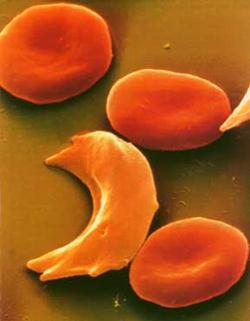
Each of us has tens of thousands of proteins, which serve a variety of functions, and each protein has a unique three-dimensional structure that specifies its function. For example, hemoglobin is a protein found in red blood cells, which plays a key role in oxygen transport; it has 4 subunits of two distinct types (2 alpha and 2 beta subunits).
is a protein found in red blood cells, which plays a key role in oxygen transport; it has 4 subunits of two distinct types (2 alpha and 2 beta subunits).
from http://gened.emc.maricopa.edu/bio/bio181/BIOBK/3_14d.jpg
|
Sickle Cell Anemia The critical relationship between protein structure and function is dramatically illustrated by sickle cell anemia, an inherited disease seen in people whose ancestors came from Africa, the Middle East, the Mediterranean, or India. In the U.S. about 4 out of every 1,000 African Americans has sickle cell disease (about 80,000 people), and about 10% carry the sickle cell trait. People with sickle cell disease an abnormal type of hemoglobin, called hemoglobin S (instead of normal hemoglobin A). Hemoglobin S differs from hemoglobin A in that the amino acid valine is found at position number 6 in the beta chain instead of the amino acid glutamate. Unlike glutamate, the side chain of valine is very non-polar and creates a sticky patch on the outside of each of the beta chains. There is a complementary sticky patch elsewhere on the hemoglobin, but it is masked as long as the hemoglobin molecules are bound to oxygen. However, if large numbers of hemoglobin molecules become deoxygenated, the sticky sites created by the abnormal valines begin to bind to the complementary sticky site on other hemoglobin molecules. This forms long aggregates of hemoglobin that distort the red blood cell and give it a characteristic sickle shape. This causes red cells to aggregate and impairs their ability to circulate through small blood vessels (arterioles and capillaries), and it also makes them fragile, shortening their life span and leading to anemia. |
Proteins as Enzymes
Some proteins function as enzymes, i.e., proteins that catalyze specific biochemical reactions. Enzymes facilitate biochemical reactions and speed them up enormously, making them as much as a million times faster. There are thousands of enzymes, and each type facilitates a specific biochemical reaction. In other words, a given enzyme only acts on specific reactant molecules (substrates) to produce a specific end product or products. The diagram below illustrates enzymatic cleavage of the disaccharide lactose (the substrate) into the monosaccharides galactose and glucose.
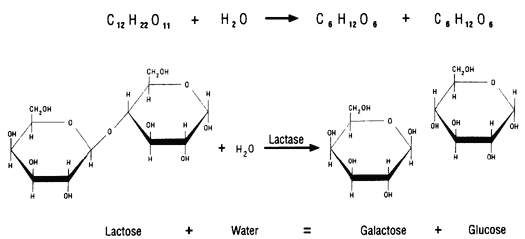
Source: http://www.indiana.edu/~ensiweb/lessons/tp.milk3.html
The three-dimensional shape of an enzyme will include a very specific binding site that the substrate will fit into very precisely, in much the same way that a key fits a specific lock.
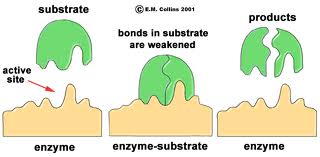
Once the substrate is bound the enzyme cleaves the substrate and the products are released. While this cartoon illustrates cleavage of a substrate, many enzymes synthesis new biochemicals by binding two substrates together to form a new product. A particular cell may have thousands of distinct enzymes catalyzing many different reactions.
The short video below illustrates the basics of how an enzyme works.
Sources: http://youtu.be/V4OPO6JQLOE
Biochemical reactions may require a whole series of steps, each of which is catalyzed by a separate enzyme. A good example is the series of reactions by which glucose is metabolized to create cellular energy in the form of ATP (adenosine triphosphate).
- First there is a series of reactions that convert glucose into two molecules of pyruvate as shown below. These reaction are referred to as "glycolysis" or as anaerobic metabolism (since they don't require oxygen). There is net production of a small amount of ATP from these reactions.
- Then, pyruvate then feeds into the citric acid cycle (also known as the Kreb's cycle or tricarboxylic acid cycle). The subsequent metabolism of pyruvate generates even more net ATP than glycolysis through the reaction illustrated below. The steps highlighted in yellow are the ones that ultimately go on to generate ATP in the cell's mitochondria.
These reactions are illustrated in the figure below.
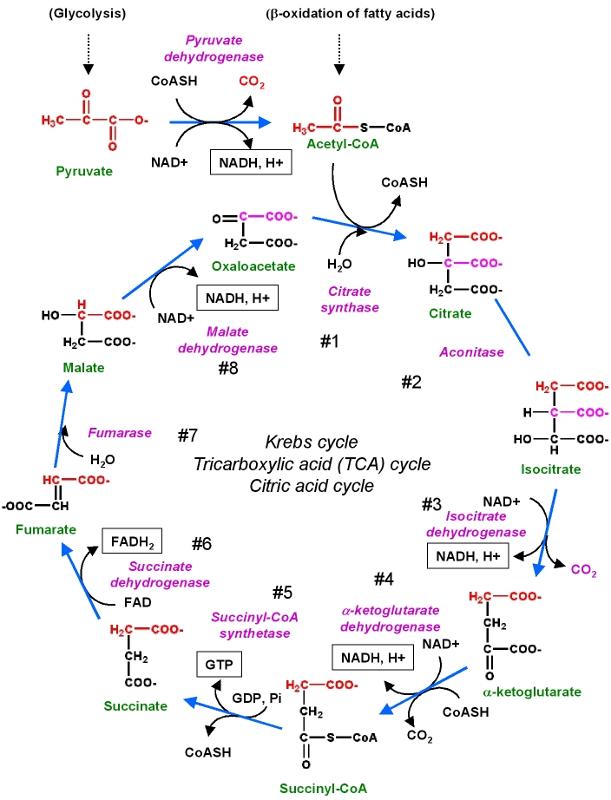
Source: http://chemwiki.ucdavis.edu/Core/Biological_Chemistry/Metabolism/Kreb's_Cycle
Lysozyme - A Defensive Enzyme
The illustration on the right shows the protein lysozyme (red, white, blue, and gray amino acids), which is an important defensive enzyme found in tears, saliva, and mucus. Lysozyme's function is to break down the polysaccharides (sugar polymers) that are components of bacterial cell walls. Initially, lysozyme is synthesized as a single long polypeptide chain, but it folds in a characteristic way to form a globular protein with a characteristic pocket. A bacterial polysaccharide (shown in green) binds to lysozyme because it fits precisely into the pocket in the same way that a key fits into a lock. Once this specific binding has occurred, lysozyme destroys the bacterial polysaccharide by cleaving it into pieces.
(red, white, blue, and gray amino acids), which is an important defensive enzyme found in tears, saliva, and mucus. Lysozyme's function is to break down the polysaccharides (sugar polymers) that are components of bacterial cell walls. Initially, lysozyme is synthesized as a single long polypeptide chain, but it folds in a characteristic way to form a globular protein with a characteristic pocket. A bacterial polysaccharide (shown in green) binds to lysozyme because it fits precisely into the pocket in the same way that a key fits into a lock. Once this specific binding has occurred, lysozyme destroys the bacterial polysaccharide by cleaving it into pieces.
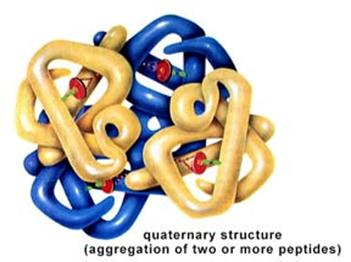
Antibodies are Proteins
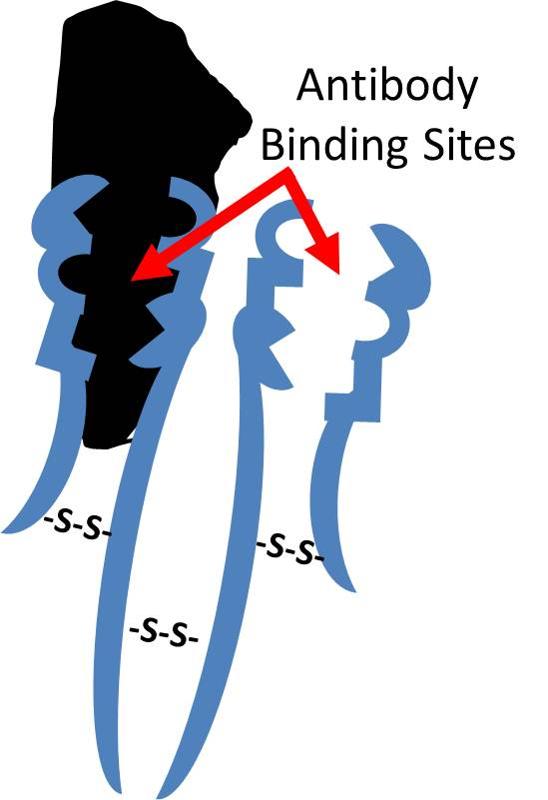 Antibodies are defensive proteins that have binding sites whose three-dimensional structure allows them to identify and bind to very specific foreign molecules. By binding to foreign proteins they can help neutralize them and tag them, facilitating their engulfment and removal by defensive cells. IgG antibodies have a quaternary structure with four subunits, two "light chains" and two "heavy chains." The chains are bound to one another through disulfide bridges, shown to the right as "-S-S-" bonds. After birth, each B-lymphocyte can manufacture antibodies for only one specific foreign shape. The portion of an antigen that is specifically recognized by an antibody is referred to an an "epitope." In essence, the epitope is a particular portion of an antigen that has a distinctive molecular shape that fits into the protein binding site on an antibody.
Antibodies are defensive proteins that have binding sites whose three-dimensional structure allows them to identify and bind to very specific foreign molecules. By binding to foreign proteins they can help neutralize them and tag them, facilitating their engulfment and removal by defensive cells. IgG antibodies have a quaternary structure with four subunits, two "light chains" and two "heavy chains." The chains are bound to one another through disulfide bridges, shown to the right as "-S-S-" bonds. After birth, each B-lymphocyte can manufacture antibodies for only one specific foreign shape. The portion of an antigen that is specifically recognized by an antibody is referred to an an "epitope." In essence, the epitope is a particular portion of an antigen that has a distinctive molecular shape that fits into the protein binding site on an antibody.
Watch the short video below to see an illustration of antibody action. The beginning of the video shows red and white blood cells flowing through a blood vessel. The potato-shaped objects that you see next represent viruses that begin binding to receptors on a cell. The green Y-shaped objects represent antibodies that bind to the virus. Finally, the Medusa-like structure represents a white blood cell that engulfs the antibody-tagged virus and destroys it.
Structural Proteins
There are also structural proteins, which are frequently long and fibrous, such as silk, keratin in hair, and collagen in tendons and ligaments.
Source: http://www.sdsc.edu/ScienceAlive/reel6/collagen.gif
Contractile Proteins
There are contractile proteins, such as actin and myosin, that provide movement in muscles and movement within single cells.
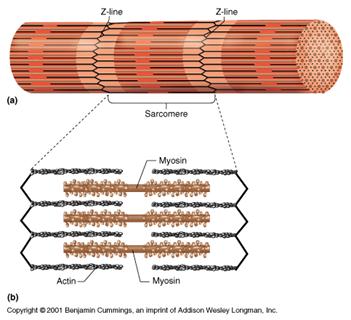
Source: http://www.bmb.psu.edu/courses/bisci004a/muscle/musc-img/myofibril.jpg
Signal Proteins
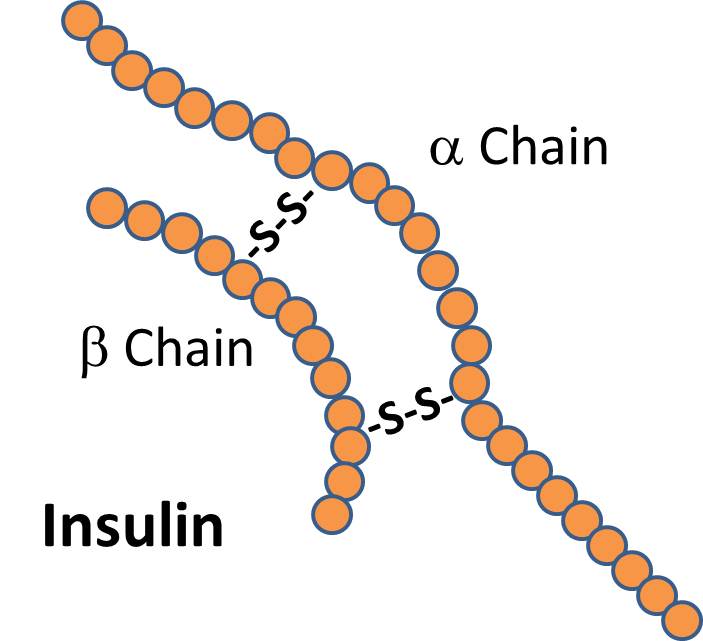 There are signal proteins, such as the hormone insulin, which consists of two polypeptide chains linked together with disulfide (two sulfur) bridges.
There are signal proteins, such as the hormone insulin, which consists of two polypeptide chains linked together with disulfide (two sulfur) bridges.
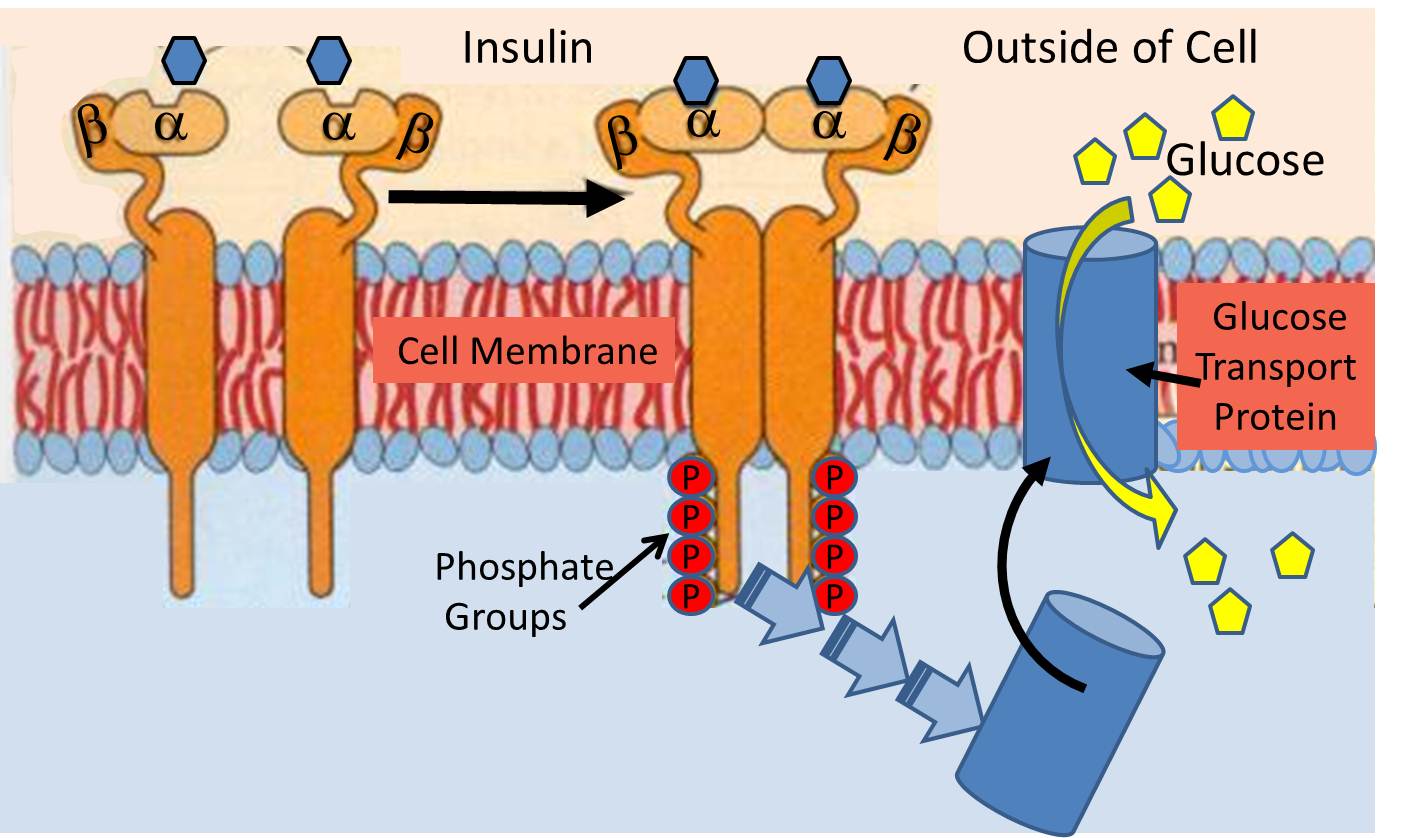 The insulin receptor (a recognition protein) is embedded in the cell membranes of muscle, fat cells and certain types of other cells. Its function is to facilitate their uptake of glucose from the blood stream through special glucose transport proteins that are normally present inside the cell in an inactive form. For example, in muscle cells, the glucose transporter is called "GLUT4". When the insulin molecule binds to the alpha subunits of the receptor, it triggers a chain reaction within the cytosol (the interior of the cell) that activates GLUT4 and causes it to be translocated and inserted into the cell membrane.
The insulin receptor (a recognition protein) is embedded in the cell membranes of muscle, fat cells and certain types of other cells. Its function is to facilitate their uptake of glucose from the blood stream through special glucose transport proteins that are normally present inside the cell in an inactive form. For example, in muscle cells, the glucose transporter is called "GLUT4". When the insulin molecule binds to the alpha subunits of the receptor, it triggers a chain reaction within the cytosol (the interior of the cell) that activates GLUT4 and causes it to be translocated and inserted into the cell membrane.
See http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html for a Flash model on insulin action.
Transportation Across the Cell Membrane
With the exception of simple diffusion, proteins are also essential for moving polarized or charged molecules and large molecules across cell membranes.
Simple Diffusion
Small molecules like oxygen and carbon dioxide can diffuse across the lipid bilayer of the cell membrane. The direction of movement depends on the concentration gradient. Substances with higher concentration inside the cell (e.g., CO2) will diffuse out of the cell toward the side with lower concentration. Substances in higher concentration outside the cell (e.g., O2) will diffuse to the inside of the cell, i.e., down the concentration gradient.
However, many other molecules cannot cross cell membranes by simple diffusion and require specialized mechanisms for movement across membranes. A variety of transport proteins, frequently aggregates of protein subunits, provide a way of transporting charged molecules and large molecules through one of two mechanisms:
Facilitated Transport
Polar molecules and charged ions cannot cross the lipid bilayer; their transit relies on special transport channels created by proteins embedded in the cell membrane. Facilitated transport is passive in that it does not require expenditure of cellular energy, and as with simple diffusion, movement of the molecules is down a concentration gradient from high concentration to low concentration. There are specific proteins for each substance transported by this mechanism, and transit can be regulated by the cell. Molecules like glucose and amino acids are transported this way. They will bind to their carrier/transpport protein, and binding triggers a change in the shape of the carrier which moves the molecule across the membrane. Once the molecule is released, the carrier returns to its original shape (conformation).
Active Transport
Active transport also relies on transmembrane transport proteins, but this process is able to transport substances against a conentration gradient, meaning that even if the concentration of, say potassium ions, is higher inside the cell than outside, more potassium can be transported into the cell. This is because cellular energy (ATP) is expended.
Proteins, then, play an integral role in the function of a cell. Many are embedded in the cell's membranes or span the entire lipid bilayer where they play an important role in recognition, signaling, and transport.