The Biology of Aging
Introduction
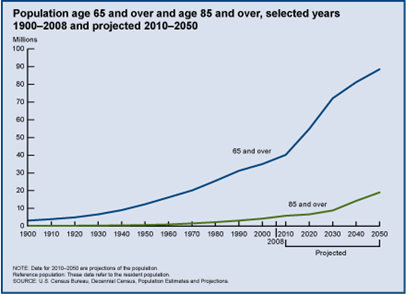 Aging is both inevitable and universal. As people age they change in a myriad of ways - biologically, psychologically and physiologically. Data from the US Census Bureau show that in 2008 there were 39 million Americans 65 years of age and older, a dramatic increase from the 3 million in 1900. In addition, as shown in the graph on the right, the populations aged 65 and over and 85 will increase dramatically in coming years, and these increases will be accompanied by a large increase in the number of people suffering from age-related diseases, such as osteoporosis and Alzheimer's disease.
Aging is both inevitable and universal. As people age they change in a myriad of ways - biologically, psychologically and physiologically. Data from the US Census Bureau show that in 2008 there were 39 million Americans 65 years of age and older, a dramatic increase from the 3 million in 1900. In addition, as shown in the graph on the right, the populations aged 65 and over and 85 will increase dramatically in coming years, and these increases will be accompanied by a large increase in the number of people suffering from age-related diseases, such as osteoporosis and Alzheimer's disease.
Despite the progress decline in function associated with aging, many elderly individuals remain cognitively sharp and physically active. These people are generally considered the fortunate recipients of "good genes," although others cite a life-long healthy life style as the key to successful aging. The rapid grow of the elderly population makes it increasingly important to understand the aging process and to promote lifestyle choices that maximize not only longevity, but also the quality of life during aging.This module will summarize what is currently known about the biology of aging, major theories of aging, physical and cognitive effects of aging, and interventions that may diminish physical and cognitive decline.
Learning Objectives
- Explain the role that the p53 protein plays in cancer and aging
- Discuss the major theories of aging including
- Discuss the potential role of caloric restriction as a possible strategy to increase life expectancy
- Summarize the physiological changes that occur during the aging process
- Explain how diet and exercise influence the aging process
The Birth and Death of Cells
The Cycle of Growth and Replication
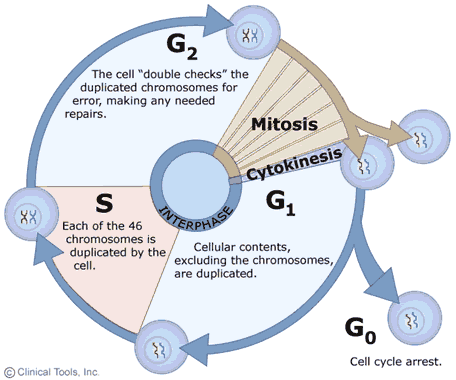 The growth and replication of cells is often described as a cyclic process with two main phases: interphase, when the cell grows and replicates DNA in preparation for cell division, and mitosis, during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.
The growth and replication of cells is often described as a cyclic process with two main phases: interphase, when the cell grows and replicates DNA in preparation for cell division, and mitosis, during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.
Regulation of the cell cycle is of critical importance to the aging process. Replication should only occur when there is a need for growth and development (in embryos and the young) or when there is a need to replace damaged or lost cells. Thus, the cycle is influenced by growth factors and by proto-oncogenes that favor replication and by anti-oncogenes that produce proteins that inhibit replication. These various factors interact to regulate the cell cycle in cells that have retained the capacity to divide. In addition, there are cellular processes that constitute checkpoints that prevent the cell cycle from proceeding if errors have occurred. The first checkpoint occurs during the G1 phase and provides an opportunity for cellular that processes to repair damaged DNA before the cell enters the S phase when replication occurs. This prevents the daughter cells from inheriting damaged DNA, which would result in mutations. There are also other checkpoints in S and G2 phases that check for damaged DNA and failure of DNA replication. The final cell cycle checkpoint occurs at the end of mitosis and checks for any chromosomes that have been misaligned. The many factors that regulate the cell cycle play an important rol in the aging process, because as cells age their capacity to replicate diminishes to the point that they are no longer able to divide. As this occurs, the ability to replace damaged or lost cells dwindles and ultimately results in declines in tissue strength and cellular and organ function that are characteristic of aging.
Aging and p53
Protein 53 (p53) is a tumor suppressor protein that is encoded by the TP53 gene. The p53 protein has been extensively studied because the gene that encodes it has been has been found to be mutated in approximately half of all human cancers. Arrest at the G1 checkpoint is mediated by p53 and is rapidly induced in response to damaged DNA. Following the arrest of cell division, p53 will also induce either cell death through apoptosis or permanent loss the ability of a cell to proliferate (senescence). The accumulation of senescent cells contributes to aging because it leads to reduced tissue renewal and repair. Several studies conducted in both mice and humans on mutant p53 genes have supported the notion that increased cancer protection by p53 can also lead to a shortened life life span.
Studies in Mice
Tyner et al studied the effects of p53 mutations in [Nature 2002;415:45-53]. Specifically, they examined the effects of either the absence of p53 or presence of a smaller than normal p53 protein in mice. In order to do this, two transgenic mouse lines were used that had either a shortened form of p53 gene (p53m, partial mutant) or a complete deletion of the p53 gene (p53-, complete deletion). They compared three groups of mice:
- group 1, p53+/p53-(deletion of one copy of p53);
- group 2, p53+/p53m (partial deletion mutant); and
- group 3, p53+/p53+ (wild type, normal).
The table below from Tyner et al. summarizes the results with respect to both cancer formation and aging ,
|
|
Genotype
|
Cancer Phenotype
|
Aging Phenotype
|
|
Group 1
|
p53+/p53- (complete deletion)
|
80% had tumors
|
Much shorter life span
|
|
Group 2
|
p53+/p53m (partial mutant)
|
None
|
Shorter life span
|
|
Group 3
|
p53+/p53+ (wild type)
|
45% had tumors
|
Normal life span
|
In group 1 (absence of p53) 80% of the mice developed tumors, while none of the mice in group 2 (shortened p53 gene) developed tumors, and 45% of the mice in group 3 (normal, wild type) developed tumors. Furthermore, group 1 had the shortest life span, group 3 (normal) had the longest life span, and group 2 had an intermediate life span. Thus, partial p53 mutation reduced the risk of developing cancer, but at the cost of a decrease in longevity. The authors also found that the group 2 mice developed phenotypes characteristic of old mice (hunchbacked spines, slow hair re-growth, etc.) earlier than the normal group 3 mice did.
Studies in Humans
Studies by Dumont et al. found that replacement of the amino acid arginine (Arg) with the amino acid proline (Pro) at position 72 on the human p53 protein decreased its ability to initiate cell death and reduced suppression of cancer. A meta-analysis by Heemst, et al. reported that carriers of this Pro/Pro genotype (two alleles with the arginine replaced by proline) have an increased risk of developing cancer compared to Arg/Arg carriers (no replacement of arginine by proline) (p<0.05). Furthermore, they conducted a prospective study of 1226 people aged 85 years or older and found that carriers of the Pro/Pro genotype had a 41% increased survival rate (p = 0.032) despite also having a 2.54 fold increase (p = 0.007) in mortality from cancer. They also found that the Pro/Pro genotype resulted in a significant decrease in mortality from all causes except for cancer and a non-significant increase in mortality from cancer. Overall, they concluded that just as in mice, human p53 protects against cancer but at a cost of longevity. [See Heemst DV, et al.: Variation in the human TP53 gene affects old age survival and cancer mortality. Experimental Gerontology 2005; 40: 11-15
Apoptosis
Apoptosis, also known as programmed cell death, is a regulated process that takes place throughout life and serves to eliminate necessary or damaged cells. Apoptosis takes place during embryonic development as a means of reshaping tissues during normal growth and development, and it provides a mechanism of eliminating worn out or damaged cells throughout life. Age-related diseases such as Alzheimer disease and Parkinson's disease have been linked to an increase in apoptosis where cells that might otherwise continue to support proper tissue functioning are eliminated. There are many pathways and proteins that regulate apoptosis, such as p53, that are able to sense cells that are damaged or no longer needed. The image below illustrates a cell undergoing apoptosis.
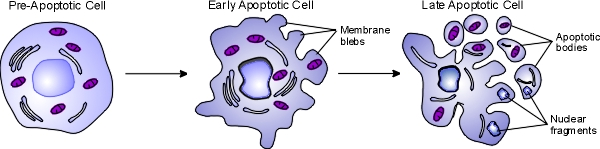
Once molecular signals trigger apoptosis, catabolic processes are initiated in the cell. Various enzymes begin to break down cellular cellular components and fragment nuclear DNA. The chromatin condenses, the cell begins to shrink and irregular bulges in the plasma membrane known as blebs form. The cell eventually breaks into several smaller pieces known as apoptotic bodies containing the cell components and the nucleus. The apoptotic bodies are then taken up by macrophages and removed.
Autophagy
Autophagy is another mechanism by which cell death can occur. Like apoptosis, it is a highly regulated process that plays a normal role in cell growth, development and homeostasis. Autophagy allows a starving cell to reallocate nutrients from unnecessary processes to more essential ones, and it also plays an important housekeeping role by removing misfolded or aggregated proteins, clearing away damaged organelles, such as mitochondria and endoplasmic reticulum, as well as eliminating intracellular pathogens. Some authors believe that mitochondrial dysfunction, which is believed to contribute to cellular aging, occurs in part because of the inability of cells to remove their damaged mitochondria. In addition, autophagy degrades proteins to enable their replacement and also degrades damaged proteins that are no longer functional. Some studies suggest that caloric restriction in rodents increases their life span by preventing the decline in autophagy that occurs with aging.
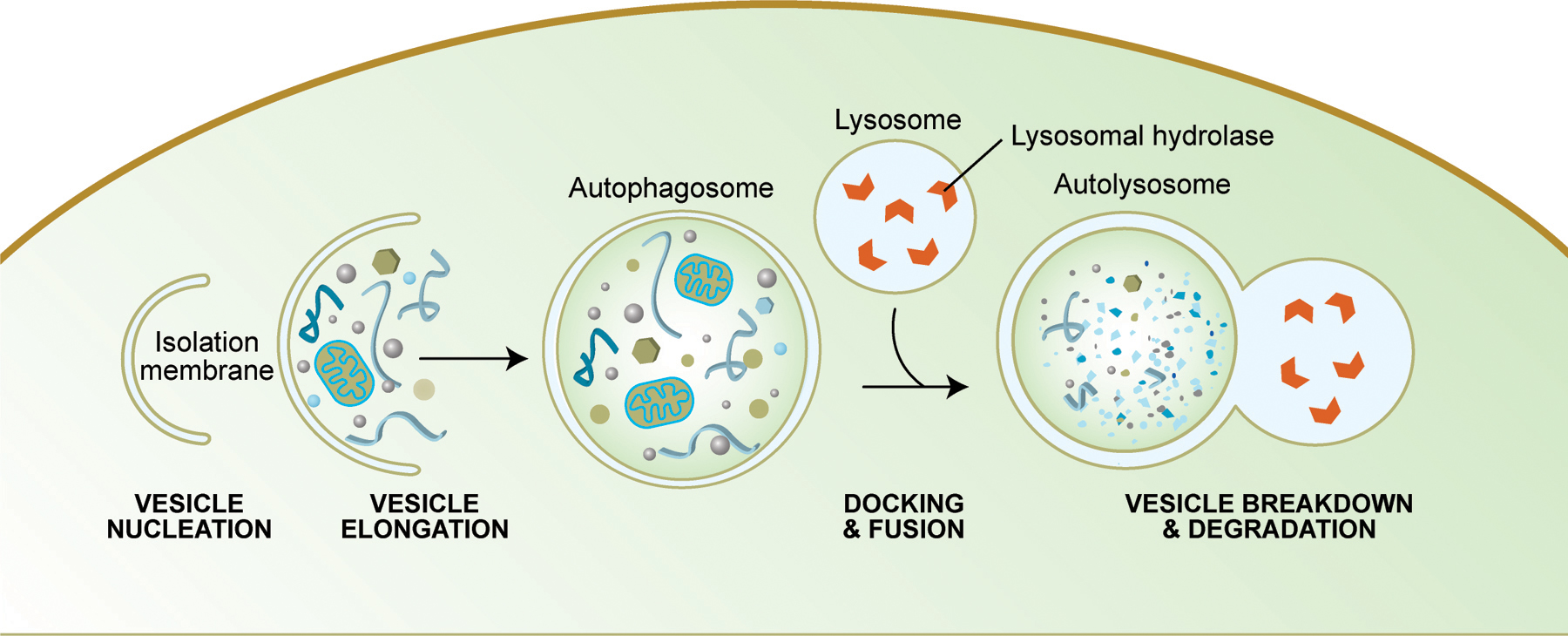
Summary of Autophagy
Source: http://www.wormbook.org/chapters/www_autophagy/autophagy.html
Theories on Aging
Why do we age? Although the question has been raised since hundreds of years ago, the mysteries that control human life span are yet to be discovered. During the last decades, many theories have been proposed to explain the process of aging, but neither of them seems to be fully satisfactory. Instead, these theories interact with each other and all of these together contribute to aging. The image below shows different mechanisms that have been proposed to explain aging. In this module, however, we will only discuss three major theories of aging: cellular senescence, DNA damage, and telomere shortening.
Cellular Senescence
Recent studies suggest that cellular senescence might be a cellular model of organismal aging. Accumulations of senescent cells were found in vivo in mammals with increasing age, and at sites of age-related pathology. [Lombard DB, Chua KF et al. :DNA Repair, Genome Stability, and Aging. Cell 2005;120 (4): 497-512.]
Cellular senescence is a state of irreversible growth arrest, which was first introduced by Hayflick and Moorhead in 1961. They found that normal human fibroblasts had a limited replicative potential and eventually entered a state of irreversible growth arrest, meaning that cells being cultured in vitro had lost the ability to divide, despite having ample space and nutrients in the culture medium. Two general models have been proposed to explain how cellular senescence may contribute to aging. First, senescent cells in tissues may accumulate to the point where the strength and functional capacity of tissues in compromised. A second model proposes that senescence in stem cells limit their regenerative potential which eventually leads to a progressive loss of tissue strength and functional capacity. Cellular senescence can be triggered by a number of mechanisms, such as telomere shortening and DNA damage an described below.
DNA Damage
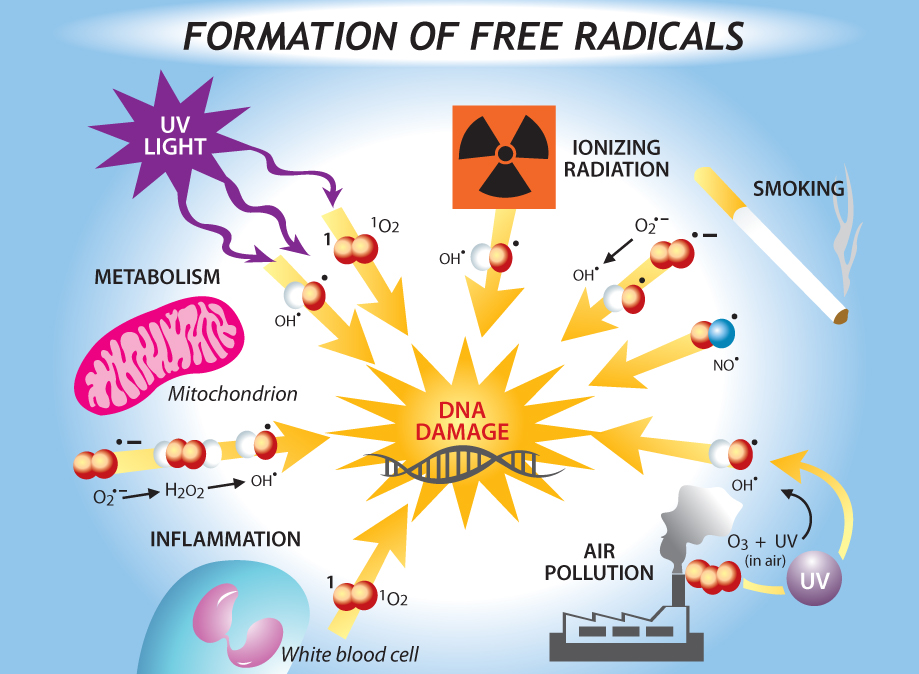 Studies showed that cellular senescence is commonly triggered by various forms of DNA damage. Mutant mice that are deficient in DNA repair show premature senescence and progeroid phenotypes, suggesting the involvement of DNA damage-induced senescence in aging. [Collado M, Blasco MA et al.:Cellular Senescence in Cancer and Aging. Cell 2007;130(2):223-233.]
Studies showed that cellular senescence is commonly triggered by various forms of DNA damage. Mutant mice that are deficient in DNA repair show premature senescence and progeroid phenotypes, suggesting the involvement of DNA damage-induced senescence in aging. [Collado M, Blasco MA et al.:Cellular Senescence in Cancer and Aging. Cell 2007;130(2):223-233.]
Sources of DNA damage include external sources, such as ionizing radiation tobacco smoke, air pollution and genotoxic drugs, and cell-intrinsic sources, such as replication errors, programmed double-strand breaks, and DNA damaging agents—reactive oxygen species (ROS). The image on the right shows a variety of sources of DNA damage. [Image source: http://churchandstate.org.uk/2013/02/the-first-person-to-live-to-150-has-already-been-born/]
Reactive oxygen species (ROS),such as superoxide anion, hydroxyl radical, hydrogen peroxide and nitric oxide, are normal byproducts of metabolism took place in mitochondria, and are believed to be one important source of DNA damage.
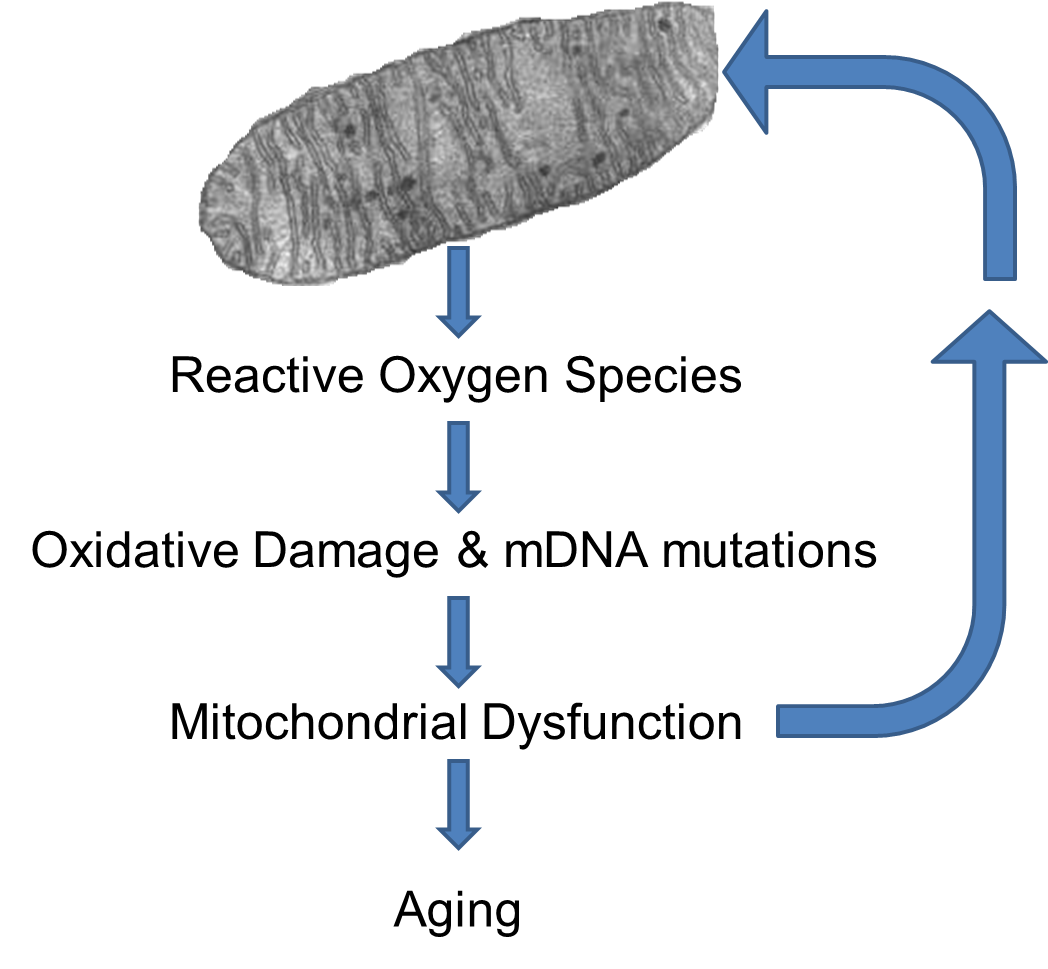
ROS can damage the mitochondria's DNA (mtDNA) and proteins, and the mutant mtDNA in turn are more liable to produce ROS byproducts. Therefore a positive feedback loop of ROS is established. With age the number of mutant mtDNA increase and the mitochondrial functions decline, leading to an increased production of ROS. [Lagouge M, Larsson NG: The Role of Mitochondrial DNA Mutations and Free Radicals in Disease and Ageing. J. Intern. Med. 2013;273(6):529-543.]
The increased generation of ROS can cause lipid peroxidation, protein damage, and several types of DNA lesions in cells. Therefore, ROS are considered important factors in the mechanisms of such diseases as diabetes, cancer, atherosclerosis, heart attacks, Alzheimer's disease, as well as in aging. Evidence has shown that species that live longer generally show higher cellular oxidative stress resistance and lower levels of mitochondrial ROS production compared to species that live shorter.
Telomere Shortening
Telomeres are repeated nucleotides sequences on the end of chromosomes that are believed to protect the DNA strands and prevent them from fusing with other strands. Telomeres lose a little bit of their length during each cell division. Since replicative DNA polymerases are not able to replicate telomeres, and telomerase (specialized DNA polymerase that could replicate telomeres) are not expressed in normal mammalian somatic cells, telomeres become too short to replicate after a fixed number of cell divisions. Eventually, the cell will stop growing and enter cellular senescence. The image below illustrates the telomere shortening as cells divide.
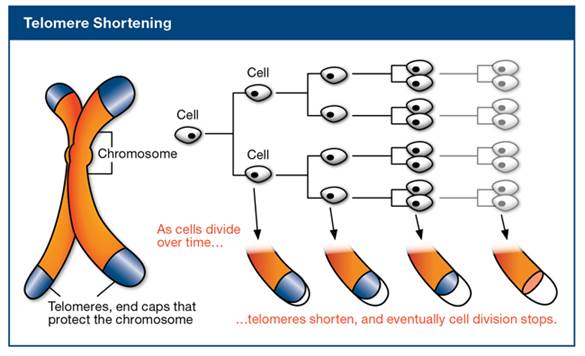
[ Image source: http://www.wholehealthinsider.com/newsletter/2012/a-genetic-solution-to-slowing-aging-and-preventing-disease/]
Telomeres are particularly susceptible to accumulation of DNA damage with age, and this damage at telomeres is restricted to be repaired because of shelterin, a multiprotein complex binding to the telomeres. Shelterin prevent the access of DNA repair proteins to the telomeres. Therefore, DNA damage at telomeres is persistent, inducing cellular senescence efficiently. [López-Otín C, Blasco MA et al.:The hallmarks of aging. Cell 2013;153 (6):1194-1217.]
The video below is a 3D animation on telomeres and telomerase (0:59min).
Calorie Restriction
Some studies have shown that calorie restriction (i.e., a 20-40% reduction of dietary caloric intake) extends life expectancy in several species ranging from yeast to rodents. One possible explanation is that caloric restriction reduced production of reactive oxygen species by mitochondria. In addition, calorie restriction induces autophagy that removes harmful proteins and organelles, thereby reducing the accumulating damage to the cell. However, this phenomenon has not yet been demonstrated in humans. For an excellent review, see Ivanova DG, Yankova TM: The free radical theory of aging in search of a strategy for increasing life span. Folia Med 2013;55(1):33-41.
Changes in Physical Function Related to Human Aging
The rate and progression of cellular aging can vary greatly from person to person. Yet, generally, over time, aging affects the cells of every major organ of the body. The summary of age-related physiological changes presented below focuses on the cardiovascular, respiratory, renal/urinary, endocrine, gastrointestinal, and musculoskeletal systems, and on the skin.

Did you know?
How does heart rate change from young adulthood to old age?
Answer

Did you know?
How does the lung function of a healthy 70 year old compare to that of a healthy 30 year old?
Answer
Cognitive Changes With Aging
How could it be that some 78 year olds can recall memories from their twenties while others cannot remember their grandchild's name? There is ample evidence that alterations in brain structure and function are intimately tied to alterations in cognitive function. In order to understand aging-related cognitive decline it is important to study the relationship between the brain and cognition. This section will provide an overview of cognitive functions of the brain and discuss cognitive decline.
Incidence rates of dementia and Alzheimer's disease in the US
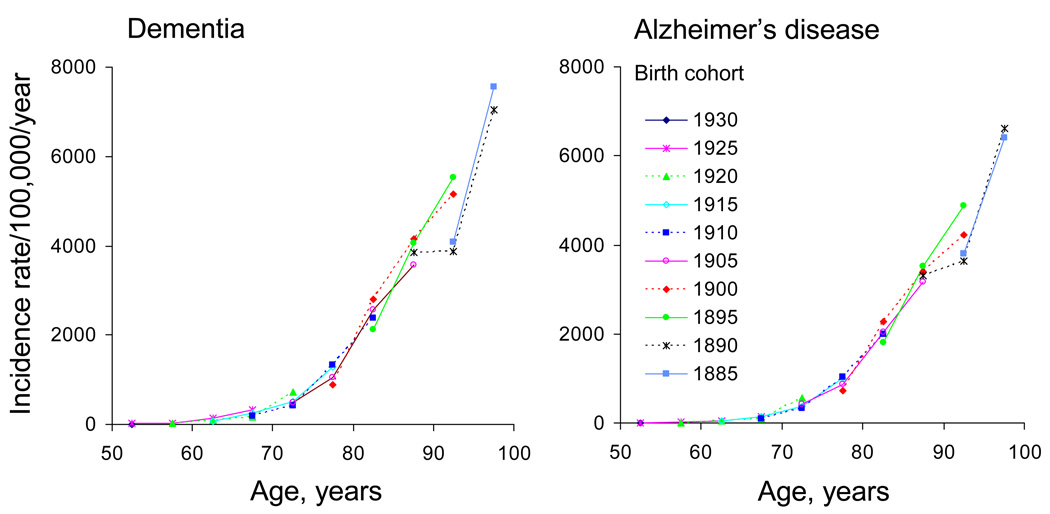
Source: Rocca WA, Petersen RC, et al.: Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimer Dement. 2011;7(1):80-93.
Many older adults complain of increased memory lapses as they age, and a major focus of research has been to try to distinguish memory declines attributable to normal aging from those that are indicative of premature, accelerated aging, particularly Alzheimer's disease.
Basic Cognitive Functions
Higher Cognitive Functions
Individual Variability in Cognitive Function
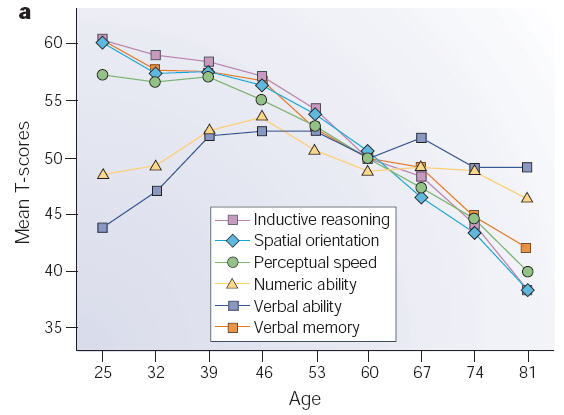 Cognitive decline is inevitable, but the extent to which it occurs and the rapidity of onset varies among individuals. There is much evidence that cognitive decline is neither uniform among people, nor is it uniform across the different cognitive functions of the brain. In other words, some 60 years olds experience worse memory loss than other 70 year olds, and one person may have excellent episodic memory but impaired executive control. This inter-individual variability is likely caused by biological, psychological, health-related, environmental, and lifestyle factors and mechanisms.
Cognitive decline is inevitable, but the extent to which it occurs and the rapidity of onset varies among individuals. There is much evidence that cognitive decline is neither uniform among people, nor is it uniform across the different cognitive functions of the brain. In other words, some 60 years olds experience worse memory loss than other 70 year olds, and one person may have excellent episodic memory but impaired executive control. This inter-individual variability is likely caused by biological, psychological, health-related, environmental, and lifestyle factors and mechanisms.
In general, however, the symptoms of cognitive decline that are associated with aging include:
- Slower inductive reasoning / slower problem solving
- Diminished spatial orientation
- Declines in perceptual speed
- Decreased numeric ability
- Losses in verbal memory
- Few changes in verbal ability
The graph on the right demonstrates how these functions decline with age. Note that there are almost no changes in verbal ability and they may even have improved with age. On the other hand, spatial orientation suffers a severe drop with age.
Dementia
Cognitive decline is a normal consequence of the age-related changes in the brain. In contrast, dementia is not a part of normal aging. Dementia is a term for a group of symptoms caused by disorders that that affect the ability to think so severely that it impairs one's ability to perform normal daily activities like eating or getting dressed. Memory loss is a common symptom of dementia, but memory loss by itself does not mean that someone has dementia. People with dementia have progressively severe problems with functions like memory and language, or they may lose their ability to solve problems. Dementia can also cause personality changes or difficulty in controlling one's emotions. Severe dementia can also result in delusions or hallucinations.
|
FamilyDoctor.org lists the following symptoms of dementia:
- Recent memory loss. All of us forget things for a while and then remember them later. People who have dementia often forget things, but they never remember them. They might ask you the same question over and over, each time forgetting that you've already given them the answer. They won't even remember that they already asked the question.
- Difficulty performing familiar tasks. People who have dementia might cook a meal but forget to serve it. They might even forget that they cooked it.
- Problems with language. People who have dementia may forget simple words or use the wrong words. This makes it hard to understand what they want.
- Time and place disorientation. People who have dementia may get lost on their own street. They may forget how they got to a certain place and how to get back home.
- Poor judgment. Even a person who doesn't have dementia might get distracted. But people who have dementia can forget simple things, like forgetting to put on a coat before going out in cold weather.
- Problems with abstract thinking. Anybody might have trouble balancing a checkbook, but people who have dementia may forget what the numbers are and what has to be done with them.
- Misplacing things. People who have dementia may put things in the wrong places. They might put an iron in the freezer or a wristwatch in the sugar bowl. Then they can't find these things later.
- Changes in mood. Everyone is moody at times, but people who have dementia may have fast mood swings, going from calm to tears to anger in a few minutes.
- Personality changes. People who have dementia may have drastic changes in personality. They might become irritable, suspicious or fearful.
- Loss of initiative. People who have dementia may become passive. They might not want to go places or see other people
|
Alzheimer's Disease
According to the National Institute of Aging, Alzheimer's disease is the most common cause of dementia among older people, and it affects an estimated 5.2 million people in the United States. Some estimates project that there will be as many as 7.7 million people with Alzheimer's disease in the US by 2030 as the Baby Boom generation ages (AARP). Alzheimer's disease is an irreversible, progressive brain disease that slowly destroys memory, language, thinking skills and the ability to plan. Over time, the ability to carry out the simplest tasks of daily living is lost. In most people with Alzheimer, symptoms first appear after age 60.
|
Comparison of Typical Age-related Memory Loss with Signs of Alzheimer's Disease
|
| Typical Age-Related Memory Loss and Changes |
Signs of Alzheimer's
|
| Making a bad decision once in a while |
Poor judgment and decision making
|
| Missing a monthly payment |
Inability to manage a budget
|
|
Forgetting which day it is and remembering later
|
Losing track of the date or the season
|
|
Sometimes forgetting which word to use
|
Difficulty having a conversation
|
|
Losing things from time to time
|
Misplacing things and being unable to retrace steps to find them
|
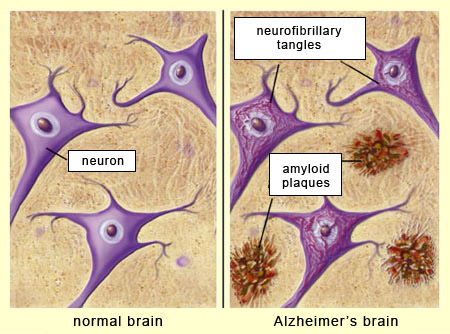
When the brains of people dying with Alzheimer's disease are examined histologically, there are two characteristic lesions seen throughout the cerebral cortex:
- Beta amyloid plaques
- Neurofibrillary tangles
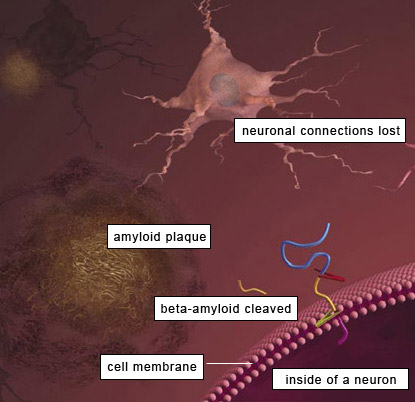
Brain tissue normally has a protein called amyloid precursor protein (APP). As part of the recycling of this protein, enzymes cleave off segments referred to as beta amyloid, which are normally removed. However, in Alzheimer's disease these sticky protein segments adhere to one another and accumulate in the spaces between neurons. As these beta amyloid plaques accumulate, they have a toxic effect on the adjacent neurons and eventually kill them.
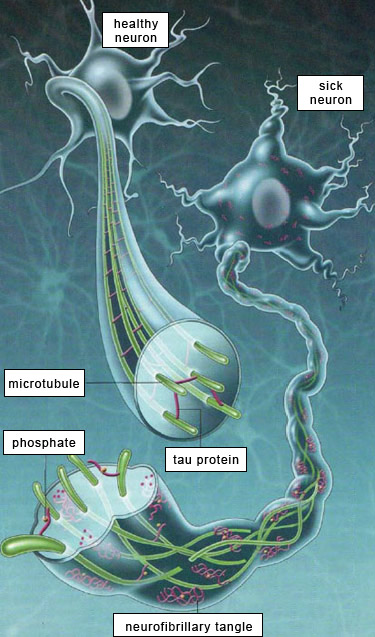
Neurofibrillary tangles are abnormalities in the microtubules inside brain neurons. Microtubules are polymers of proteins that form long cylindrical tubules within cells. These provide structure and provide a scaffold for transport of substances within cells. The microtubules are stabilized by special proteins called "tau" proteins. With Alzheimer's disease the tau protein separate from the microtubules and clump together to form intracellular tangles. The loss of functioning microtubules disrupts intracellular transport of vesicles, nutrients, and organelles.
|
|
|
|
Images from McGill University - "The Brain From Top to Bottom"
http://thebrain.mcgill.ca/flash/d/d_08/d_08_cl/d_08_cl_alz/d_08_cl_alz.htm |
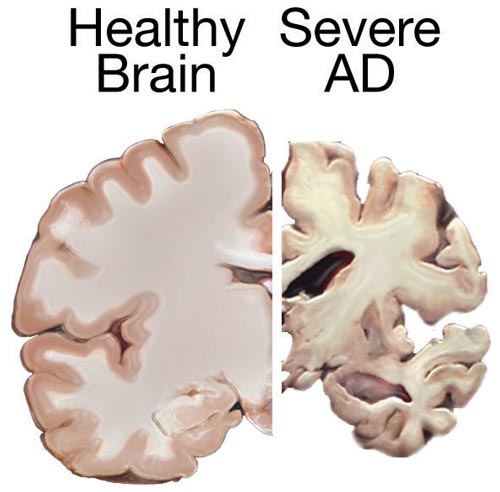 During the early stages of Alzheimer's disease (up to ten years), there are no symptoms, but the plaques and neurofibrillary tangles accumulate and progressive kill more and more brain cells. As an increasing number of neurons die, affected brain regions begin to shrink. In the final stages of Alzheimer's disease, the damage is widespread, and brain tissue has shrunk significantly. (See the image to the right from the National Library of Medicine.) In late stage Alzheimer's disease, individuals lose the ability to carry on a conversation or respond to their environment. On average patients diagnosed with Alzheimer's disease die about 8-10 years after symptoms become noticeable to others. There is no cure for Alzheimer's disease, although several drugs have been developed which may slow the progression of disease.
During the early stages of Alzheimer's disease (up to ten years), there are no symptoms, but the plaques and neurofibrillary tangles accumulate and progressive kill more and more brain cells. As an increasing number of neurons die, affected brain regions begin to shrink. In the final stages of Alzheimer's disease, the damage is widespread, and brain tissue has shrunk significantly. (See the image to the right from the National Library of Medicine.) In late stage Alzheimer's disease, individuals lose the ability to carry on a conversation or respond to their environment. On average patients diagnosed with Alzheimer's disease die about 8-10 years after symptoms become noticeable to others. There is no cure for Alzheimer's disease, although several drugs have been developed which may slow the progression of disease.
The captioned video below [4:22 min] from the National Institute on Aging provides an overview of Alzheimer's disease.
Inside the Brain: Unraveling the Mystery of Alzheimer's Disease.
Promoting Healthy Aging
Aging and death are, of course, inevitable, but despite the progress decline in function associated with aging, many elderly individuals remain cognitively sharp and physically active. This section explores lifestyle choices that appear to be associated with increased longevity and enhanced quality of life in older individuals.
Social Circles & Support Systems
Several studies have suggested that maintaining a large network of personal relationships, and regularly engaging in social and productive activities is associated with a decreased risk of cognitive and physical decline. Similarly, social disengagement, defined as having very few or no social relationships, is a strong risk factor for cognitive and physical decline.
One specific study on community-dwelling centenarians found similar results. Centenarians have confidantes, daily visitors, and someone from their family, typically their children, or a friend acting as a caregiver. These individuals help with meal preparation, sickness, or disability and therefore help keep the centenarian relatively healthy. This solidifies the fact that having friends, family members, caregivers or confidantes is very important for living a long healthy live.
Smoking
Smoking shortens people's lives by an average of 14 years. More people die from smoking than from HIV, illegal drug use, alcohol use, motor vehicle injuries, suicides, and murders combined. Smoking causes 1 of every 5 deaths in the United States each year from the following ailments:
- Lung disease: Smoking damages the lungs and airways, sometimes causing chronic bronchitis and emphysema.
- Heart disease: Smoking increases your risk of heart attack and stroke;
- Cancer: Smoking can lead to cancer of the lung, mouth, larynx, esophagus, stomach, liver, pancreas, kidney, bladder, and cervix;
- Respiratory problems: Smokers are more likely than nonsmokers to get the flu, pneumonia, or other infections that can interfere with breathing;
- Osteoporosis: Smokers have a greater chance of developing osteoporosis than nonsmokers.
The good news about smoking, though, is that it is never too late to quit. Quitting smoking at any time provides serious and immediate health benefits. Those who quit smoking generally experience improvements in breathing and control of high blood pressure. Cessation o smoking bring a rapid reduction in risk of stroke and myocardial infarction and a slower reduction in the risk of cancer.
Leisure Activities
Many studies suggest that engaging in leisure activities regularly reduces cognitive decline, especially with respect to Alzheimer's disease and dementia due to severe vascular disease. The extent to which risk declines is to some extent related to the frequency and type of leisure activity participation. Leisure activity can be divided into two subgroups: physical activities and cognitive activities.
Regular participation in cognitive activities is associated with a reduced risk of dementia. Participating in physical activities has beneficial effects on the brain. It keeps a person sharp by enhancing resistance to insults, promoting plasticity, increasing the levels of neurotrophic factors, and promoting overall health. However, there is no convincing evidence that physical activity reduces the risk of dementia.
In addition to reducing the risk of dementia, cognitive activities also improve performance on cognitive tasks. These tasks include perceptual discrimination, visual search, recognition, recall, spatial perception. Memory training through computer training, memory tapes or games (i.e., chess and bridge) can also be very helpful. These trainings typically teach mnemonic strategies, concentration, attention, relaxation, personal insight, self-monitoring, motivation, feedback, and problem solving that have succeeded in improving memory performance.
Diet
Diet plays a major role in the prevention and management of debilitating cognitive and physical conditions, especially for aging individuals. Consuming too much or too little dietary substance can have detrimental effects. In undernutrition, which is different from calorie restriction discussed in the "Theories of Aging" section, individuals do not consume the needed amount of protein and nutrients for optimal health. Undernutrition can lead to some types of dementia and can accelerate osteoporosis. On the other hand, overnutrition increases the risk of heart disease, diabetes, osteoporosis, gallstone disease, hypertension, cancer, and sleep apnea.
Healthy centenarians did not engage in overnutrition,, and they generally avoided weight loss diets and large fluctuations in body weight. Instead, they ate a balanced diet, which included the regular consumption of breakfast, avoided dietary cholesterol, drank milk, consumed 20-30% more carotenoids and vitamin A.
The USDA has an excellent web site that provides a wealth of information about healthy eating and physical activity. See http://www.choosemyplate.gov/.
Effects of Exercise on Physical Health
Exercise has a beneficial impact on physical health. Higher levels of physical activity have been associated with:
- An increase in survival
- A delay in progression of disability
- A delay in the loss of functional ability
- An improvement in balance and strength
- A reduction in incidence of falls and fractures
- Higher quality of life
- An improvement in mood
These improvements occur because exercise moderates muscular, myocardial, skeletal muscle, oxidative stress, and inflammation associated with aging patterns. Exercise also has an important role in the prevention and retardation of osteoporosis, the reduction of fracture risk, prevention of colon and breast cancer and the prevention of obesity. For those with chronic disease or recovering from acute illnesses, exercise programs are an important part of the management. Exercise training must be tailored to an individual's goals and capacities pertain to age, gender, and medication use. However, it is recommended that older adults have 30-60 min of moderate intensity (5-6 on a 0-10 pain scale) exercise training per day or 75-100 min per day of vigorous (7-8 on the pain scale 3-5 times a week. In addition, the elderly should either have 2 days a week of moderate or vigorous intensity weight training and calisthenics and 2 days per week of flexibility training. Stretching is also recommended for each activity.
Effects of Exercise on Cognitive Health
The protective effects of regular exercise on cognitive health were documented in a recent comprehensive analysis of 15 studies including over 33,000 subjects who were followed for up to 12 years. The study concluded that all levels of physical activity suggest a significant and consistent protection against the occurrence of cognitive decline. Low to moderate levels of physical activity conveyed a 35% reduction in risk for cognitive decline. While individuals with the highest levels of physical activity were a striking 38% less likely to show signs of cognitive decline over time compared to those with very-low activity levels.
Aging Intervention and Future Research on Stem Cells
Aging is accompanied by a decline in cellular regenerative capacity of all tissues and organs. Decline in the regenerative activities of these tissues can be attributed to an age-related decline in stem cell function. As a result, scientists and researchers are delving into the world of stem cell therapy to look for possible answers to treat, and hopefully one day cure, some of these age related disorders. However, before a cure for such disorders can be found, we have to understand how our environment, physical age, cellular age, and stem cell age react with one another. Regardless of this fact, significant progress has been made in the field of stem cell research and more promising research is to come.
References
Biology of Aging
Journal Articles
- Cui J, et al.: Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One 2013; 8(7): e69720
- Dumont P, et al.: The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 2003; 33(3): 357-365
- Glick D, et al.: Autophagy: cellular and molecular mechanisms. J Pathol 2010; 221(1): 3-12
- Heemst DV, et al.: Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol 2005; 40: 11-5
- Mays-Hoopes LL: Aging and Cell Division. Nature Education 2002; 3(9):55
- Shen J, et al.: Programmed cell death and apoptosis in aging and life span regulation. Discov Med 2009; 8(43):223-226
- Tyner SD, et al.: p53 mutant mice that display early ageing-associated phenotypes. Nature 2002; 415: 45-53
- Warner HR: Aging and regulation of apoptosis. Curr Top Cell Regul 1997; 35: 107-121
- Zhang Y, et al.: Ageing and apoptosis. Mech Ageing Dev 2002; 123(4): 245-260
- Zong WX, et al.: Necrotic death as a cell fate. Genes Dev 2006; 20(1): 1-15
Websites
- http://www.agingstats.gov/Main_Site/Data/2010_Documents/Population.aspx
- http://publications.nigms.nih.gov/insidethecell/chapter5.html#6
- http://biology.clc.uc.edu/courses/bio104/mitosis.htm
- http://www.nia.nih.gov/sites/default/files/biology_of_aging.pdf
Books
- The Cell: A Molecular Approach, Geoffrey M. Cooper and Robert E. Hausman, Sinauer Associates, Inc., 5thEdition, 2009
Major Theories of Aging
- Jeyapalan JC, Sedivy JM: Review
Cellular senescence and organismal aging. Mech Ageing Dev 2008; 129(7-8): 467-74 http://www.ncbi.nlm.nih.gov/pubmed/18502472
- Lombard DB, Chua KF et al.: Review DNA Repair, Genome Stability, and Aging. Cell 2005; 120(4): 497-512 http://www.sciencedirect.com/science/article/pii/S0092867405001029
- Collado M, Blasco MA et al.: Review Cellular Senescence in cancer and aging. Cell 2007; 130(2): 223-33 http://www.ncbi.nlm.nih.gov/pubmed/17662938
- Ivanova DG, Yankova TM: The free radical theory of aging in search of a strategy for increasing life span. Folia Med 2013; 55(1): 33-41 http://www.ncbi.nlm.nih.gov/pubmed/23905485
- Lagouge M, Larsson NG: The Role of Mitochondrial DNA Mutations and Free Radicals in Disease and Ageing. J Intern Med 2013; 273 (6): 529-543 http://www.ncbi.nlm.nih.gov/pubmed/23432181
- López-Otín C, Blasco MA et al.:The Hallmarks of Aging. Cell 2013;153 (6): 1194-1217 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836174/
- Pallauf K, Rimbach G: Review Autophagy, polyphenols and healthy ageing. Ageing Res Rev 2013; 12(1): 237-52 http://www.ncbi.nlm.nih.gov/pubmed/22504405
Physical Aging
- Buckwalter JA, Heckman JD, Petrie DP: An AOA: Critical Issue: Aging of the North American Population: New Challenges for Orthopaedics. The Journal of Bone and Joint Surgery 2003;85(4):748-758 .
- Daniels K, et al. "Exercise: a vital means to moderate cardiovascular aging." Aging Health 2013;9(5):473 .
- Drozdowski L, Thomson AB: Aging and the intestine. World Journal of Gastroenterology 2006;12(47):7578-7584 .
- Gerontology Center. The Georgia Centenarian Study. The University of Georgia
- Isik B, et al. Development of skin aging scale by using dermoscopy" Skin Research & Technology 2013;19(2): 69-74 .
- Lamberts SW, Van den Veld AW, and Van der Lely A-J: The Endocrinology of Aging. Science 1997:278(5337):419-424 .
- McLeod RP, et al. A lifetime of well skin care: practical recommendations for clinicians and patients. Skin & Allergy News 2013;44(9):pS16 .
- North BJ, and Sinclair DA: The intersection between aging and cardiovascular disease. Circulation Research 2012;110:1097-1108 .
- Piccin D, and Morshead CM: Potential and pitfalls of stem cell therapy in old age. Disease Models & Mechanisms 2010;3:421-425 .
- Raiser SN and Vincent KR: The aging musculoskeletal system and obesity-reslated considerations with exercise. Aging Research Review 2012;11(3):361-373 .
- Rughwani N: Normal anatomic and physiologic changes with aging related disease outcomes: a refresher. Mount Sinai Journal of Medicine 2011;78: 509-514.
- Russell RM: Changes in gastrointestinal function attributed to aging. American Society for Clinical Nutrition 1992;55:1203S-7S .
- Woo J: Relationship among diet, physical activity and other lifestyle factors and debilitating diseases in the elderly. European Journal of Clinical Nutrition 2000;54(3):S143.
Cognitive Aging
- Alzheimer's Association. "Dementia with Lewy Bodies." Accessed November 30, 2013. < http://www.alz.org/dementia/dementia-with-lewy-bodies-symptoms.asp>.)
- Alzheimer's Association. "Vascular Dementia." Accessed November 30, 2013. < http://www.alz.org/dementia/downloads/topicsheet_vascular.pdf>.)
- Alzheimer's Association. "What is Alzheimer's?" Accessed November 30, 2013. < http://www.alz.org/alzheimers_disease_what_is_alzheimers.asp>.)
- Anderton BH: "Aging of the Brain." Mechanisms of Ageing and Development 2002: 123 (7): 811-817.
- Esir MM, et al.: "Aging and Dementia." Greenfield's Neuropathology 1997;153-233.
- Godefroy O, et al: "Age-Related Slowing: Perceptuomotor, Decision, or Attention Decline?" Experimental Aging Research, Apr-Jun2010; 36(2): 169-189.
- Jernigan, T.L. et al.: "Effects of Age on Tissues and Regions of the Cerebrum and Cerebellum." Neurobiology of Aging 2001 ; 22: 581-594.
- Lu PH et al.: "Age Related Slowing in Cognitive Processing Speed is Associated with Myelin Integrity in a Very Healthy Elderly Sample." J. Clin. Exp. Neuropsychol. 2011;3310):1059-1068.
- McGill University. "The Brain from Top to Bottom: Cognitive Losses Associated with Alzheimer's." Accessed November 30, 2013. < http://thebrain.mcgill.ca/>.
- Morley JE: "The top 10 hot topics in aging." J Gerontol A Biol Sci Med Sci. 2004 Jan; 59(1):24-33.
- National Center for Learning Disabilities: "What is Executive Function?" Accessed November 29, 2013. < http://www.ncld.org/types-learning-disabilities/executive-function-disorders/what-is-executive-function>.
- National Institute on Aging, National Institutes of Health. "Frontotemporal Disorders: Information for Patients, Families and Caregivers." Accessed November 30, 2013. < http://www.nia.nih.gov/alzheimers/publication/frontotemporal-disorders>.
- Rocca WA, et al: "Trends in the Incidence and Prevalence of Alzheimer's Disease, Dementia and Cognitive Impairment in the United States." Alzheimer's & Dementia 2011; Vol. 7: 80-93
- Rosling C: "Serotonin." University of Bristol. Accessed November 29, 2013. < http://www.chm.bris.ac.uk/motm/serotonin/home1.htm>.
- Sowell ER, et al.: "Mapping cortical change across the human life span." Nat. Neurosci. 2003; 6(3): 309-315.
- Web MD. "Serotonin: 9 Questions and Answers." Accessed October 15, 2013 <http://www.webmd.com/depression/features/serotonin>.
Interventions
- CDC. "CDC - Fact Sheet - Tobacco-Related Mortality - Smoking & Tobacco Use."Smoking and Tobacco Use. N.p., 1 Aug. 2013. Web. 6 Dec. 2013. <http://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/>.
- Life Extension Foundation for Longer Life. "Age Related Cognitive Decline." Accessed October 15, 2013. <http://www.lef.org/protocols/neurological/age_related_cognitive_decline_02.htm>.
- Williams, KN, et al.: "Interventions to Reduce Cognitive Decline in Aging." J. Psychosoc. Nurs. Ment. Health Serv. 2010; 48(5): 42-51.
 Aging is both inevitable and universal. As people age they change in a myriad of ways - biologically, psychologically and physiologically. Data from the US Census Bureau show that in 2008 there were 39 million Americans 65 years of age and older, a dramatic increase from the 3 million in 1900. In addition, as shown in the graph on the right, the populations aged 65 and over and 85 will increase dramatically in coming years, and these increases will be accompanied by a large increase in the number of people suffering from age-related diseases, such as osteoporosis and Alzheimer's disease.
Aging is both inevitable and universal. As people age they change in a myriad of ways - biologically, psychologically and physiologically. Data from the US Census Bureau show that in 2008 there were 39 million Americans 65 years of age and older, a dramatic increase from the 3 million in 1900. In addition, as shown in the graph on the right, the populations aged 65 and over and 85 will increase dramatically in coming years, and these increases will be accompanied by a large increase in the number of people suffering from age-related diseases, such as osteoporosis and Alzheimer's disease. The growth and replication of cells is often described as a cyclic process with two main phases: interphase, when the cell grows and replicates DNA in preparation for cell division, and mitosis, during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.
The growth and replication of cells is often described as a cyclic process with two main phases: interphase, when the cell grows and replicates DNA in preparation for cell division, and mitosis, during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.

 Studies showed that cellular senescence is commonly triggered by various forms of DNA damage. Mutant mice that are deficient in DNA repair show premature senescence and progeroid phenotypes, suggesting the involvement of DNA damage-induced senescence in aging. [Collado M, Blasco MA et al.:Cellular Senescence in Cancer and Aging. Cell 2007;130(2):223-233.]
Studies showed that cellular senescence is commonly triggered by various forms of DNA damage. Mutant mice that are deficient in DNA repair show premature senescence and progeroid phenotypes, suggesting the involvement of DNA damage-induced senescence in aging. [Collado M, Blasco MA et al.:Cellular Senescence in Cancer and Aging. Cell 2007;130(2):223-233.]



 Cognitive decline is inevitable, but the extent to which it occurs and the rapidity of onset varies among individuals. There is much evidence that cognitive decline is neither uniform among people, nor is it uniform across the different cognitive functions of the brain. In other words, some 60 years olds experience worse memory loss than other 70 year olds, and one person may have excellent episodic memory but impaired executive control. This inter-individual variability is likely caused by biological, psychological, health-related, environmental, and lifestyle factors and mechanisms.
Cognitive decline is inevitable, but the extent to which it occurs and the rapidity of onset varies among individuals. There is much evidence that cognitive decline is neither uniform among people, nor is it uniform across the different cognitive functions of the brain. In other words, some 60 years olds experience worse memory loss than other 70 year olds, and one person may have excellent episodic memory but impaired executive control. This inter-individual variability is likely caused by biological, psychological, health-related, environmental, and lifestyle factors and mechanisms.


 During the early stages of Alzheimer's disease (up to ten years), there are no symptoms, but the plaques and neurofibrillary tangles accumulate and progressive kill more and more brain cells. As an increasing number of neurons die, affected brain regions begin to shrink. In the final stages of Alzheimer's disease, the damage is widespread, and brain tissue has shrunk significantly. (See the image to the right from the National Library of Medicine.) In late stage Alzheimer's disease, individuals lose the ability to carry on a conversation or respond to their environment. On average patients diagnosed with Alzheimer's disease die about 8-10 years after symptoms become noticeable to others. There is no cure for Alzheimer's disease, although several drugs have been developed which may slow the progression of disease.
During the early stages of Alzheimer's disease (up to ten years), there are no symptoms, but the plaques and neurofibrillary tangles accumulate and progressive kill more and more brain cells. As an increasing number of neurons die, affected brain regions begin to shrink. In the final stages of Alzheimer's disease, the damage is widespread, and brain tissue has shrunk significantly. (See the image to the right from the National Library of Medicine.) In late stage Alzheimer's disease, individuals lose the ability to carry on a conversation or respond to their environment. On average patients diagnosed with Alzheimer's disease die about 8-10 years after symptoms become noticeable to others. There is no cure for Alzheimer's disease, although several drugs have been developed which may slow the progression of disease.