Frances Oldham Kelsey
A Case Study
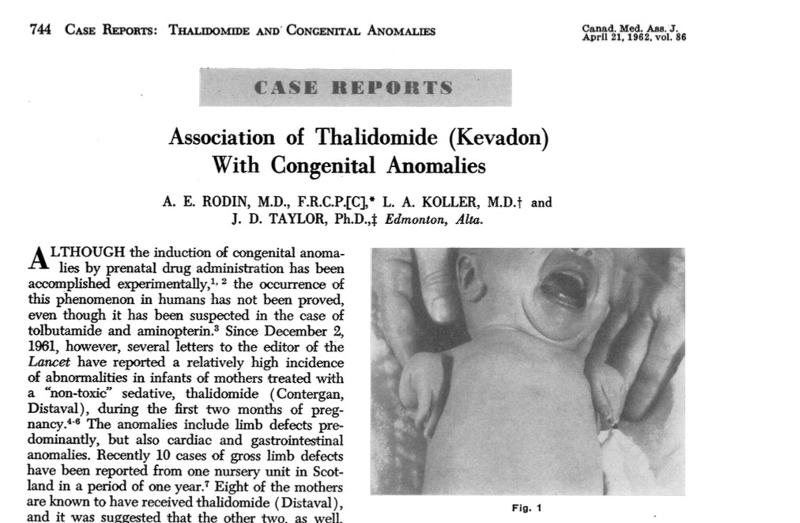
Kevadon (or better known by its generic name, Thalidomide) was a widely used drug in the 1950s to support sleep and to treat nausea in pregnant women.1 The Grünenthal Group in Germany created Thalidomide in 1953 and the first paper on its pharmacological effects was published in 1956.2 Reports noted its superiority to comparator drugs and its nontoxic elements.3 Thalidomide was licensed for use in the UK in 1958 and reached the Food and Drug Administration for marketing approval in September 1960.4 The drug application to the FDA, however, never passed, as reviewers required more data to prove its safety. In 1961, reports began to emerge in Germany and the United Kingdom that women taking thalidomide during pregnancy gave birth to children with severe birth defects.5 In 1961, the Australian doctor, William McBride wrote to the Lancet Medical Journal regarding an observed increase in the number of deformed babies born at his hospital, all of which took thalidomide during pregnancy.6 By late 1961, several case reports and further evidence surfaced across Europe, Britain, Canada, and the Middle East linking Thalidomide to children born with flipperlike arms and legs and other defects including deformities of ears, eyes, and cardiac and gastrointestinal irregularities.7

After successfully completing this module, the student will be able to:

Frances Oldham Kelsey was born in British Columbia in 1914. Her parents wanted her to receive an education as good as her older brother's, and they sent her to an all boys private school, where she was the only girl enrolled for many terms.8 Frances received her Bachelor of Science and Master of Science degrees from McGill University, Montreal in 1934 and 1935. McGill University began to accept female students in 1884 due to Donald A. Smith's $120,000 endowment to the University on condition that women and men receive the same standard of education.9 Kelsey later pursued her PhD in pharmacology from the University of Chicago in 1938. Thereafter she received an offer from the University of Chicago for a faculty position in a letter addressed to Mr. Oldham. The pharmacology professor, Eugene Geiling, misconstrued "Frances" as the masculine counterpart to "Francis", and hired her without an interview. After realizing the mistake, Kelsey asked a McGill University professor whether she should accept the offer:
'When a woman took a job in those days, she was made to feel as if she was depriving a man of the ability to support his wife and child, Dr. Kelsey told the New York Times in 2010. But my professor said: 'Dont be stupid. Accept the job, sign your name and put "Miss" in brackets afterward.'"10
While on faculty at the University of Chicago, Kelsey earned her medical degree in 1950. She later joined the FDA in 1960, where she was responsible for evaluating applications for licenses to market new drugs.11 During her first month on the job, the Thalidomide case crossed her desk. The Cincinnati-based drug company, William S. Merrell, had licensed the drug and stood to profit substantially upon its approval from the FDA. Since the drug was already widely distributed across Canada, Europe, and other parts of the world, Merrell and FDA colleagues expected an easy and quick approval process. However, Kelsey found Merrell's application incomplete and insufficient to warrant the drugs approval.12
In a 1960 memo, Kelsey described the material submitted by Merrell as "an interesting collection of meaningless pseudoscientific jargon apparently intended to impress chemically unsophisticated readers."13 When Kelsey repeatedly rejected Merrell's application, Merrell representatives complained to Kelsey's bosses at the FDA and held protests through phone calls, letters, and repeated office visits. Kelsey requested information on clinical and animal studies on the drugs toxicity and its safety during pregnancy. By the end of 1961, however, reports emerged that associated the drug with severe birth defects such as phocomelia, a birth defect in which infants are born without limbs or with severely deformed limbs.14

European countries subsequently withdrew the drug from its markets and Merrell revoked its application to the FDA. While Kelsey prevented what could have been thousands of children born with such deformities in the U.S., the Merrell Company had distributed the drug for investigational purposes to more than 1,000 physicians. According to the FDA, however, there were 17 confirmed cases of phocomelia in the U.S. and 9 other likely cases, compared to approximately 8,000 children worldwide that suffered from phocomelia, the majority died before birth.15 Another report, however, indicates that the "clinical trials" these physicians participated in resulted in the distribution of the drug to approximately 20,000 patients across the nation, over 3,000 of which were of childbearing age.16 Poor record keeping made it difficult to trace the actual number of individuals impacted.
A Revolution in Drug Development17
In 1962, President Kennedy presented Kelsey with an award for "Distinguished Federal Civilian Service" for her work in preventing significant thalidomide-related birth defects in the United States. The overwhelming public concern of the Thalidomide case also led to the support of the Kefauver-Harris Amendments, which established stringent safeguards for drug approval.18

The Kefauver-Harris Amendments were originally intended to reform drug pricing and marketing, as Senator Kefauver believed that "patients were paying too much and [were] being misled by extravagant advertising claims."19 After the Thalidomide tragedy, however, Kefauver reintroduced his legislation to include FDA drafted provisions that would prevent another alarming situation like that of thalidomide.20
Signed into law by President Kennedy on October 10, 1962, the amendments "established a framework that required drug manufacturers to prove scientifically that a medication was not only safe, but effective."21 Prior to this law, the FDA operated under lax conditions and lacked well-established standards around the conduct of clinical trials. The amendments also required FDA approval of a drug before marketing, requiring that all patients give informed consent and that companies keep records of where investigational records are sent.22 Kelsey and her colleagues at the FDA thereafter established three distinct phases for human clinical trials among other rules for human protections, which are now the standard worldwide.23
After the thalidomide incident, Kelsey became the head of the FDA's Investigational Drug Branch (now the Division of Scientific Investigations) where she made further progress in clinical trial regulation, including establishing the three phases of clinical research that are used as the gold standard worldwide. She enforced FDA regulation, created new regulations in the 1970s, and conducted oversight visits to study sites to ensure high quality study data. The former commissioner of health affairs at the FDA, Stuart Nightingale, MD, noted that Kelsey played a key role in what is now considered "good clinical practices" globally. She remained active in her efforts to push for good clinical practice throughout her work in the 1990s and continued to serve as an in house expert on teratology and advisor to FDA staff working on such issues throughout her career.24
Labeled as "Thalidomide babies," the children are now in their 50s and have lived the majority of their lives with such deformities. Two victims in the U.S. share their stories in the Huffington Post where they reflect, "we were deemed dysfunctional, broken, damaged from the start." They share their experiences attending school, driving without 'adaptors, engaging in hobbies, battling with the decision to use prosthetics, and continually working to uncover the truths of the thalidomide story.

