The Adaptive Immune System
"Presentation" of Antigens
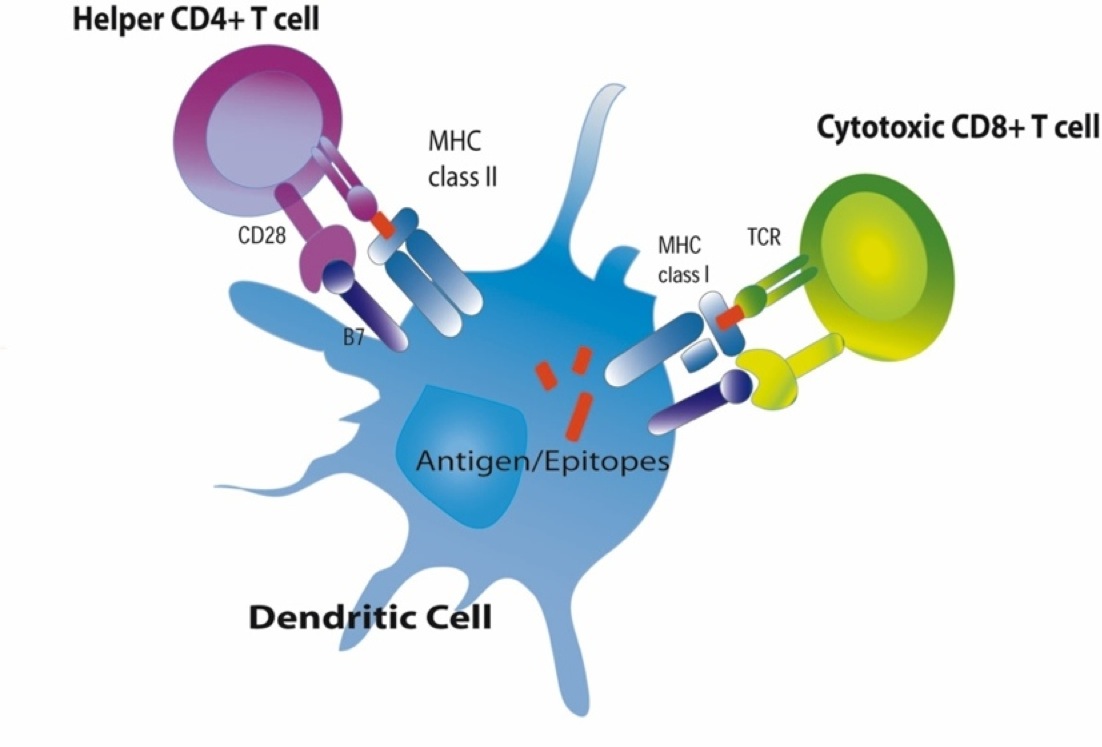
The MHC molecules also play a major role in directing the adaptive immune system. There are two major classes of MHC molecules: MHC class I and MHC class II.
MHC Class I Molecules
 MHC I glycoproteins are present on all of the nucleated cells in the body (they are not present on red blood cells or platelets). The function of MHC class I molecules is to take pieces of any protein synthesized within the cell
MHC I glycoproteins are present on all of the nucleated cells in the body (they are not present on red blood cells or platelets). The function of MHC class I molecules is to take pieces of any protein synthesized within the cell and "present" them on the cell surface. Cells are constantly turning over cell proteins, removing old ones and replacing them with new ones. As part of this process, recycled proteins are broken into small fragments called peptides, and these are sent to the endoplasmic reticulum where some of the peptide fragments bind to a groove on the surface of newly-synthesized MHC class I molecules. The MHC-peptide complex is then transported to the cell surface and inserted into the cell membrane so that the peptide fragment is "presented" to the exterior of the cell where it is accessible to lymphocytes.
and "present" them on the cell surface. Cells are constantly turning over cell proteins, removing old ones and replacing them with new ones. As part of this process, recycled proteins are broken into small fragments called peptides, and these are sent to the endoplasmic reticulum where some of the peptide fragments bind to a groove on the surface of newly-synthesized MHC class I molecules. The MHC-peptide complex is then transported to the cell surface and inserted into the cell membrane so that the peptide fragment is "presented" to the exterior of the cell where it is accessible to lymphocytes.
This mechanism becomes extremely valuable if a cell becomes infected with a virus or if it undergoes malignant transformation (becomes cancerous). Viruses are not able to reproduce on their own; they must use a host cell's synthetic "machinery" to make copies of the viral components, including viral proteins. Some of these viral proteins will also be broken into peptide fragments and combined with MHC class I molecules on the cell surface.
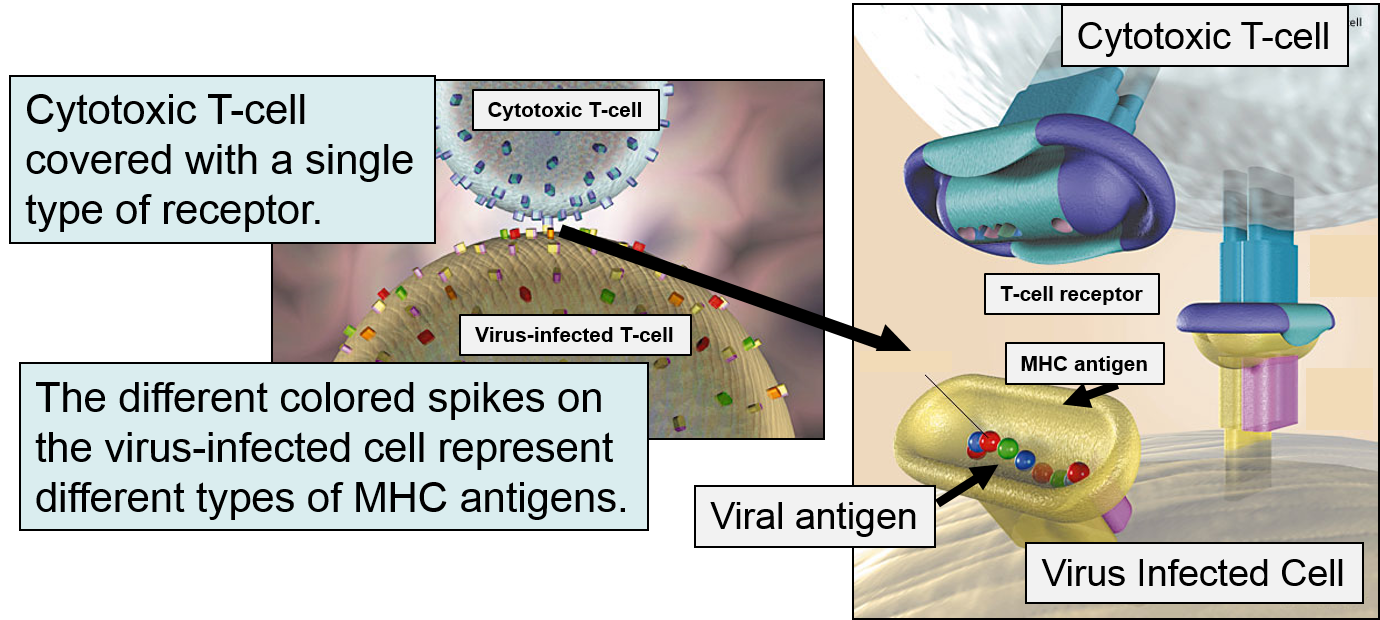
If a cytotoxic T-cell (CD+8) encounters a peptide fragment that has a complementary shape to its receptor, it will bind to the MHC-peptide complex and secrete cytotoxic molecules that penetrate the infected cell and kill it, effectively ending the production of more virus particles. Consequently, MHC Class I proteins work to present the types of proteins being synthesized within a cell, so that they can be monitored by lymphocytes in order to destroy cells producing unfamiliar proteins, i.e., cancer cells or virus-infected cells.
MHC Class II Molecules
MHC II glycoproteins are only present on macrophages, dendritic cells, and B cells. All three of these cell types are capable of phagocytosis, and their function is to engulf antigens that originate from outside the cell, e.g., on bacteria. After the exogenous antigens are broken down, the resulting peptide fragments are bound to MHC II molecules and presented on the cell surface. These cells will typically migrate to nearby lymph nodes where helper T cells with receptors that match the antigen have a greater opportunity to encounter the antigen and bind to it. When this occurs, the helper T cell lymphocytes become activated and begin to release cytokines that attract other cells to the area of infection in order to destroy the infectious agents with that antigenic material. B lymphocytes can also engulf foreign antigens, break them down, and display the resulting peptides on MHC II molecules on their surface. If a helper T lymphocyte binds to a peptide fragment on the surface of a B cell, it stimulates the B cell to divide repeatedly and differentiate into plasma cells which produce antibody against the antigenic material.
antigens are broken down, the resulting peptide fragments are bound to MHC II molecules and presented on the cell surface. These cells will typically migrate to nearby lymph nodes where helper T cells with receptors that match the antigen have a greater opportunity to encounter the antigen and bind to it. When this occurs, the helper T cell lymphocytes become activated and begin to release cytokines that attract other cells to the area of infection in order to destroy the infectious agents with that antigenic material. B lymphocytes can also engulf foreign antigens, break them down, and display the resulting peptides on MHC II molecules on their surface. If a helper T lymphocyte binds to a peptide fragment on the surface of a B cell, it stimulates the B cell to divide repeatedly and differentiate into plasma cells which produce antibody against the antigenic material.
|
|
Source: http://www.amaltherapeutics.com/rd%20cancer%20vaccine.html |
The video below illustrates the role of macrophages in presenting antigen.
Antigens and Epitopes
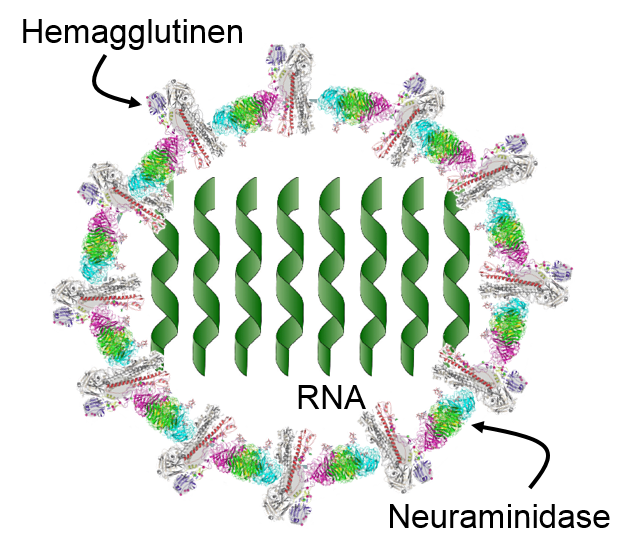
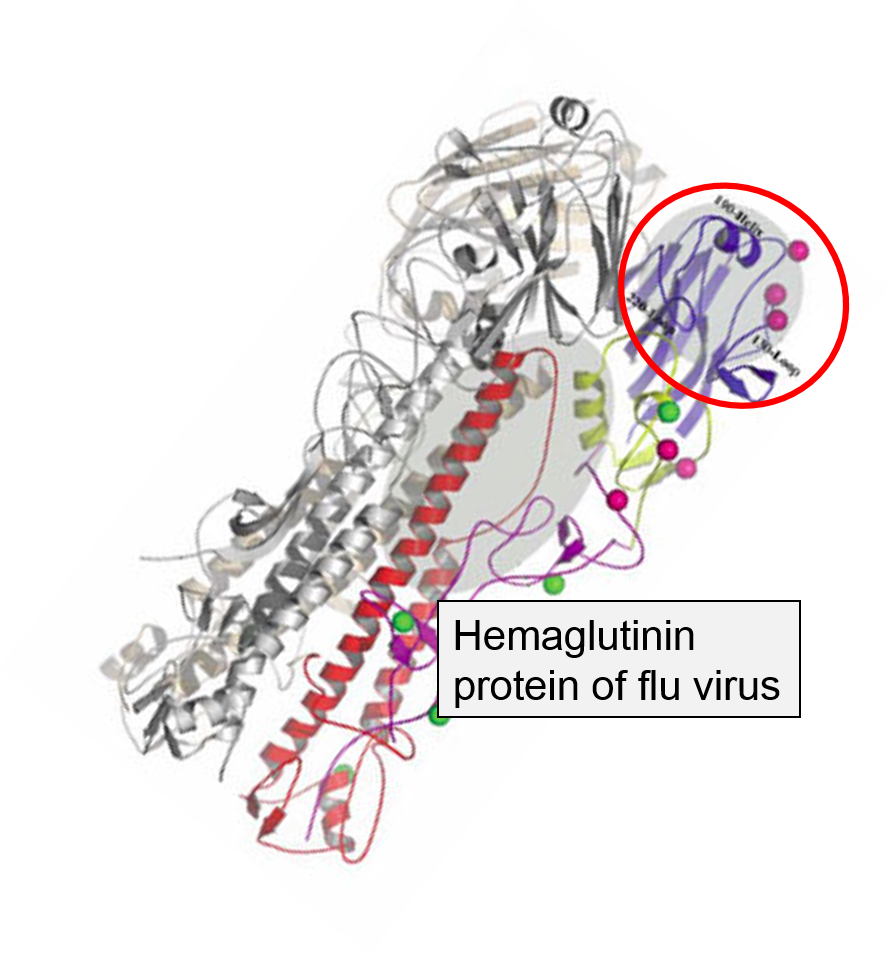
The innate immune system is triggered by PAMPs or, in the case of natural killer cells, by the absence of MHC class I molecules on a cell's surface, but the adaptive immune system is triggered by very specific molecular shapes, which are generally referred to as antigens. The illustration on the left is a representation of an influenza virus, which consists of an exterior shell of hemagglutinen and neuraminidase proteins and eight RNA strands in its core. The hemagglutinen and neuraminidase proteins are potential antigens, but there are only specific portions of these molecules that might be "recognized" by our immune system. The illustration on the right is an enlarged image of a hemagglutinen protein, and the portion of the molecule circled in red might represent a specific shape, i.e., an epitope , on the hemagglutinen molecule that would be recognized by our immune system.
, on the hemagglutinen molecule that would be recognized by our immune system.
|
An Influenza Virus |
A Hemagglutinen Molecule from an Influenza Virus |
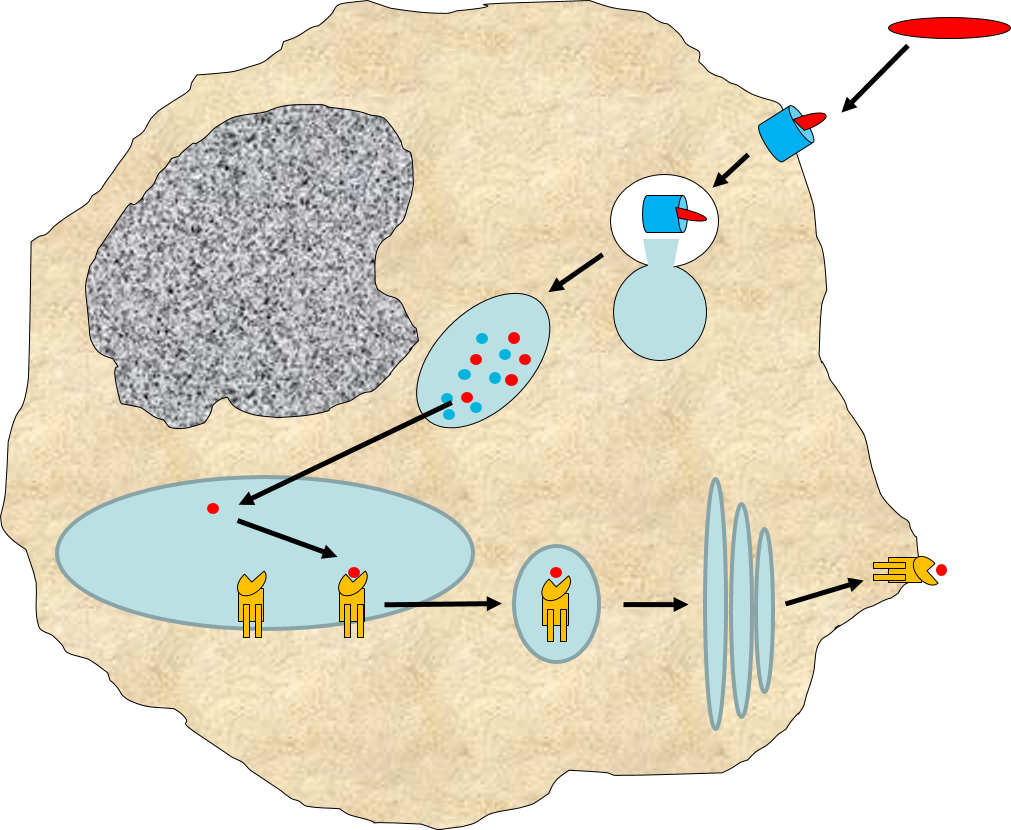
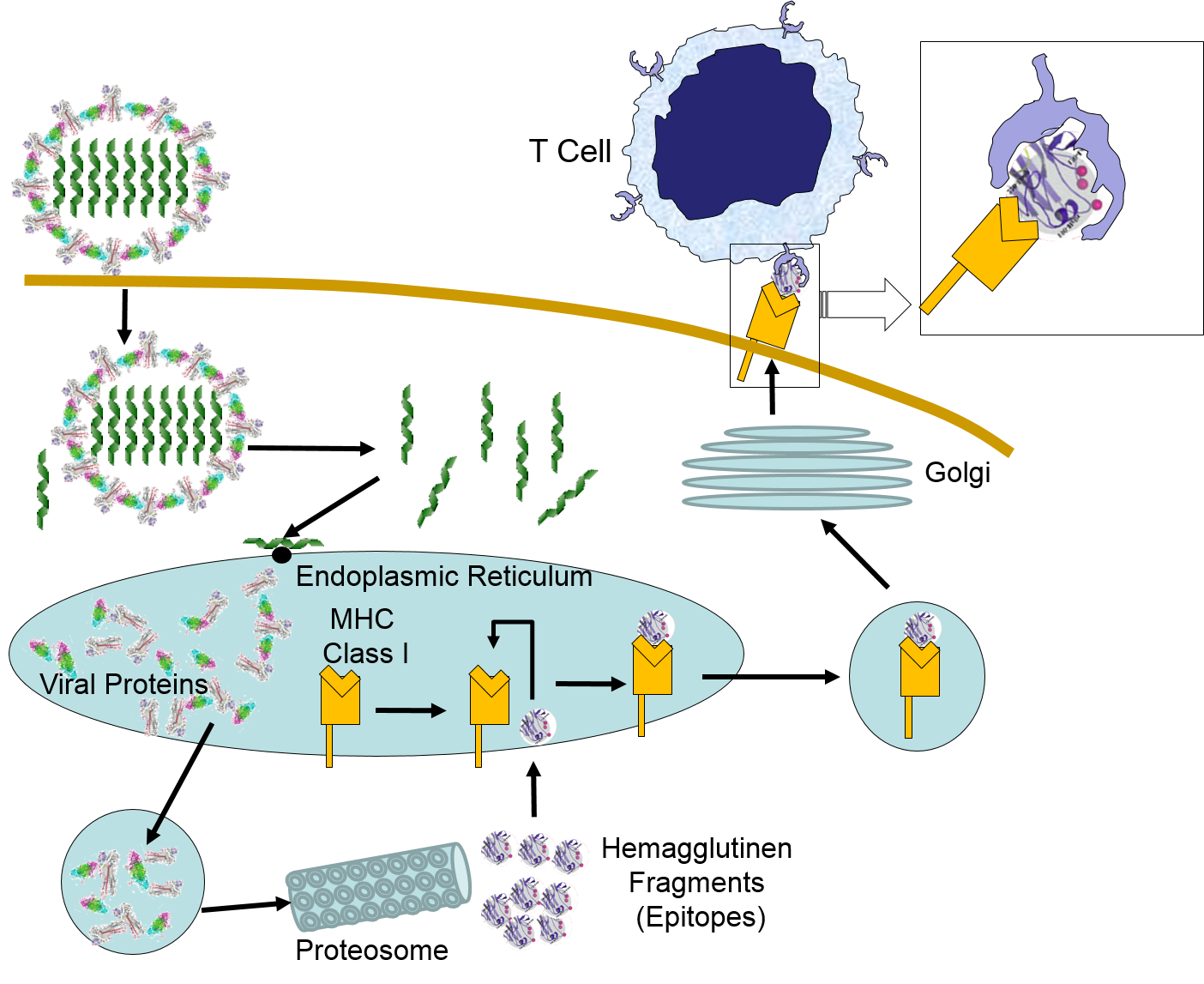
When influenza virus infects our cells (e.g., epithelial cells in our nose and throat) its protein coat disintegrates, and the viral RNA uses our ribosomes and substrates to produce more viral proteins and more copies of its DNA. However, as noted above, samples of internally synthesized proteins (including viral proteins) are broken down in proteosomes, and the fragments are complexed with MHC Class I molecules in the endoplasmic reticulum. The MHC Class I and attached fragments are then inserted into the cell membrane where the fragments are "presented" to cells of the immune system. These events are depicted in the figure below. Helper T cells with matching receptors would become activated and recruit additional lymphocytes, and cytotoxic T cells with matching receptors would bind to the cell and secrete cytotoxic molecules that penetrate the infected cell and kill it, effectively ending the production of more virus particles.
in the image below virus binds to a human epithelial cell and becomes internalized. It then sheds its protein coat and begins to replicate viral RNA and proteins uses the cells organelles and substrates. Some of the viral proteins are transported from the endoplasmic reticulum to proteosomes which break them into fragments which are bound to MHC Class I molecules. These are then transported to the cell membrane and inserted with the protein fragments "presented" to the exterior of the cell where T cells with matching receptors can bind to the fragments and become activated.

Antibodies
B lymphocytes can become activated by direct contact with a pathogen or foreign protein if they have a receptor that is complementary to an epitope on the foreign agent. Helper T cells that have become activated by antigen presentation will further stimulate the activated B cell to replicate over and over and to transform into a large clone of plasma cells that produce antibodies specific for that epitope. These antibodies are widely distributed in the circulation and can bind to the epitopes, tagging the foreign agents to facilitate its identification and destruction by phagocytic cells. The image below shows an antibody binding to a specific epitope on two virus particles. Keep in mind, however, that antibodies can similarly participate in defense against any agent or substance that has matching epitopes.
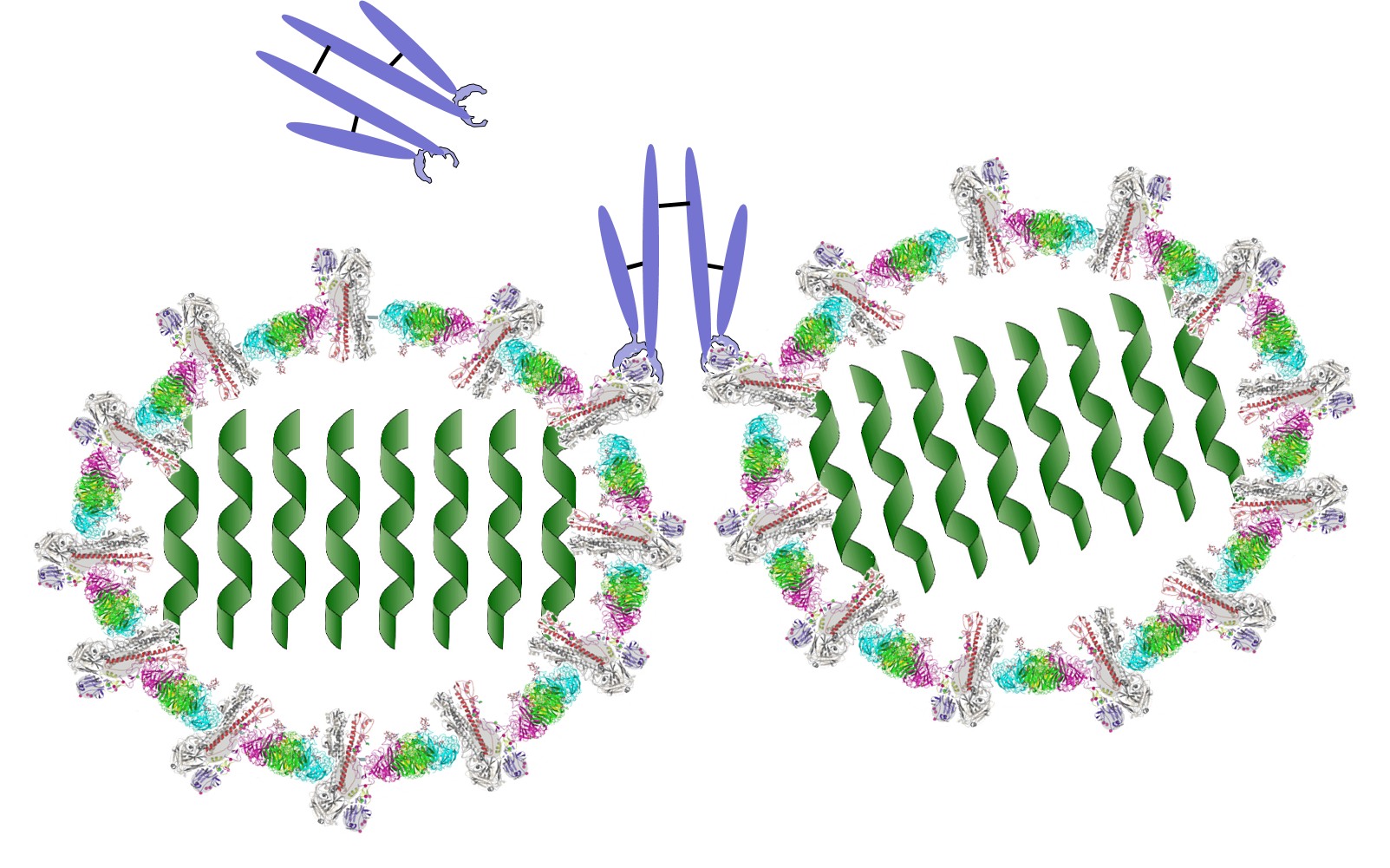
What Antibodies Do
- Opsonization: Bind to foreign antigen to tag invader and facilitate phagocytosis by leukocytes.
- Neutralize bacterial toxins by binding to them; white blood cells phagocytize the trapped toxin.
- Prevent viruses from entering cells: Antibodies can bind to viruses that have not yet entered a cell and prevent them from binding and entering.
- Activate the inflammatory response by activating the complement cascade and by stimulating mast cells to release histamine.
Classes of Antibodies
 |
Roll over the tabs to view more information. |
|
This content requires JavaScript enabled.
|
|
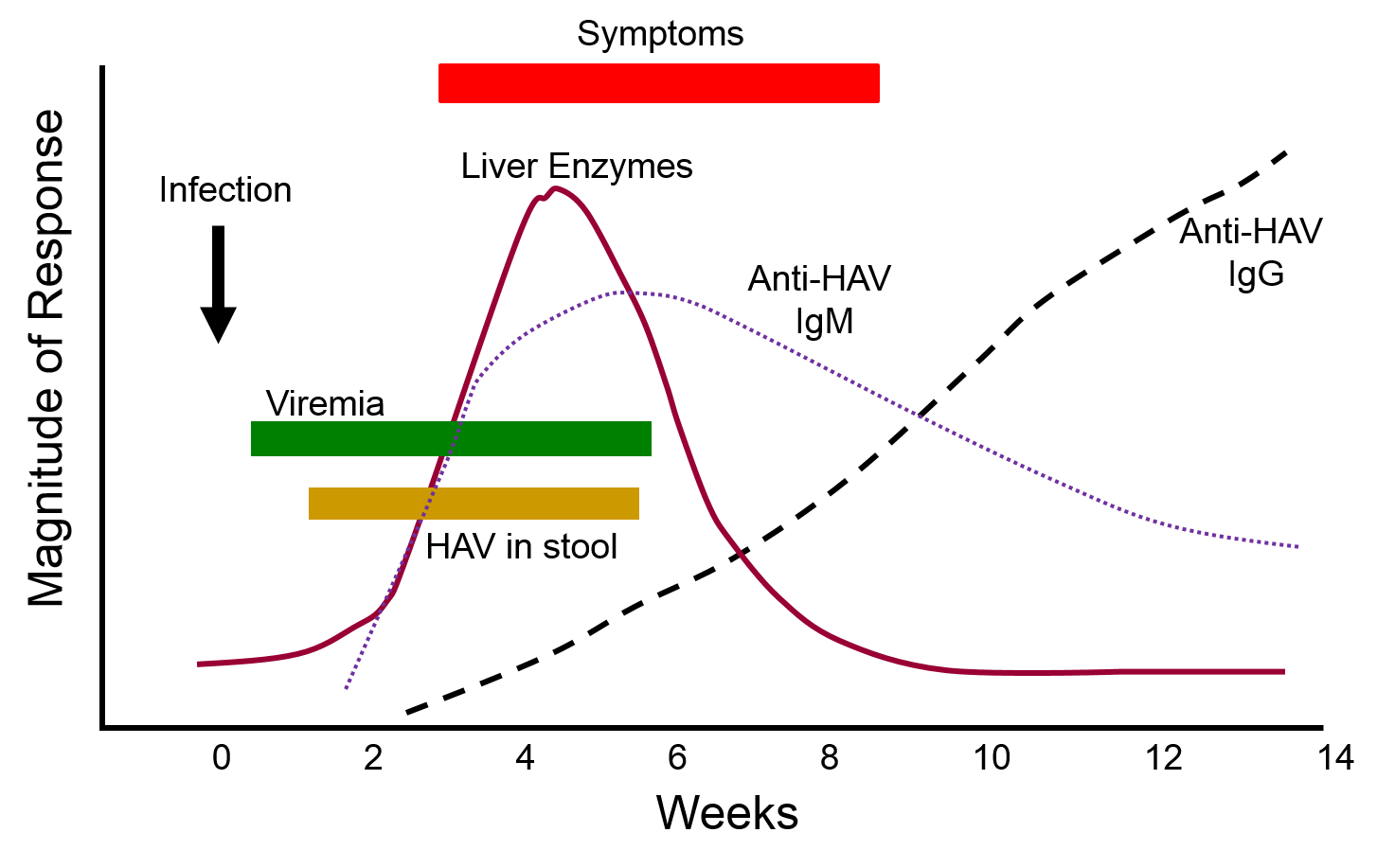
The graph below depicts the sequence of events that occur during infection with hepatitis A virus (HAV). Note, first, that the presence of virus in blood (viremia) and in stool occurs well before the onset of symptoms, making it easy for a victim to transmit the virus to others. Also, note that levels of IgM antibodies in blood rise early and then begin to decline. IgG levels rise somewhat later, but they persist for a much longer time. By measuring the titers (concentrations) of both IgM and IgG antibodies against HAV, it is possible to determine whether an individual was recently infected, or if they were infected some time ago. This information could be important in determining whether a particular food handler, for example, was responsible for an outbreak of hepatitis A.