
Influenza
Contributors
Influenza is an acute viral infection with symptoms ranging from mild to severe depending on an individual's phenotype. This virus circulates worldwide and can affect any age group. Influenza is attributed to not only social and economic disruption, but also hundreds of thousands of deaths each year. With the recent 2009 swine flu pandemic, influenza has become a major priority for governments and health agencies. This module will touch on the history of influenza, its biological basis, interventions, surveillance, and determinants of influenza.
After successfully completing this section, the student will be able to:

Influenza has the potential to become a worldwide epidemic and to affect large portions of the human population, with numerous consequences. Unlike seasonal influenza epidemics, pandemics occur irregularly and can spread quickly due to increased commerce and air travel. Also, unlike the average 36,000 deaths per year due to seasonal epidemics, pandemics can cause hundreds of thousands of deaths within the US alone. Characteristics and challenges of flu pandemics include rapid worldwide spread, health care systems that become overloaded, inadequate levels of medical supplies and manpower and economic and social disruption (Flu.gov, 2011). The figure below shows annual US mortality rates from various causes from 190900-1965. The enormous spike in deaths due to influenza and pneumonia in 1918 is impressive to say the least.
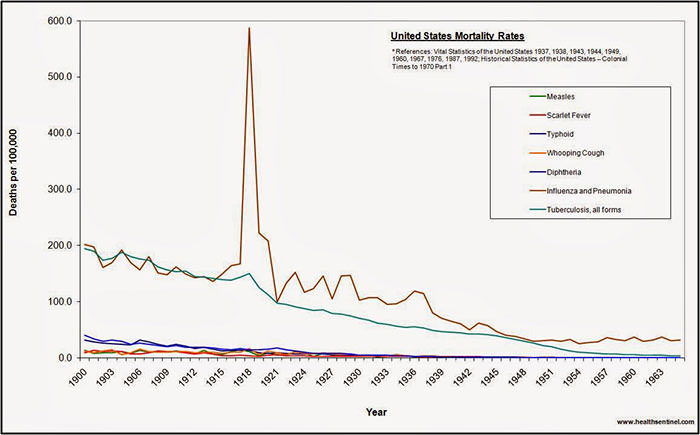
Source: https://www.learnodo-newtonic.com/spanish-flu-facts
Worldwide the great influenza of 1918 is believed to have caused at least 25-50 million deaths. Many believe it is inevitable that period influenza pandemics will continue to occur in the future. It is therefore imperative that we understand the causes and prepare for the next pandemic.
The video below (2:37 min) provides a glimplse of the devastating effects of the 1918 pandemic.
Viruses are non-living. They must reproduce by entering an animal or plant cell (i.e., a host) and using the hosts cell's organelles energy and nutrients to synthesize copies of the viral proteins and viral genetic material (DNA or RNA). Once these components have been replicated they self-assemble to form new virus particles.
The influenza virus has a nucleic core with genetic material surrounded by a membrane bound protein coat consisting of four proteins:
Within the Orthomyxoviridae family of RNA viruses there are A, B and C types of influenza viruses. The A and B viruses cause seasonal epidemics throughout the world, while the C virus only causes mild illnesses and is not thought to cause epidemics.
The specific steps that occur during infection with an influenza virus were summarized by Hate (Hate, 1999).
Influenza has two predominant mechanisms of transportation: person-to-person and fomites.
The influenza virus can have a wide range of symptoms varying from mild to severe. (Hunt, 2010).
Secondary bacterial infection (usually Streptococcus pneumoniae, Staphylococcus aureus, Hemophilus influenza) can also occur.
Non-pulmonary complications of influenza include the following:
Avian, Swine, and Human Interaction
New influenza strains of influenza virus arise continually as a result of two mechanisms that alter the genetic code in the viral RNA. These mechansims are referred to as genetic drift and genetic shift.
Genetic Drifts— Small changes in genetic material called point-mutations. The antigenic proteins of influenza may be subject to the small mutations and not recognized by antibodies (Hunt, 2010). (Explain this more)
Genetic Shifts result from reassortment of genetic material between vastly different strains of influenza viruses. (This needs to be explained much better.) This can involve the creation of new H and N proteins on the virus coat (Hunt, 2010). If the new viral proteins are sufficiently different from pre-existing viral proteins, there may be limited immune recognition by humans. As a result, the majority, if not all, of the human population may be susceptible to the new viral form. In addition, reassortment may also result in a very virulent new strain. (Hunt, 2010).
All three organisms can be infected with influenza virus. Pigs are susceptible to both human and avian virus strands. They act as an intermediate step in combining avian, human, and swine influenza genetic material. This occurs when pigs become infected with both human and avian virus. The influenza RNA strands reassort while in the pig to form new combinations of avian, swine, and human strands. In turn, these new influenza viruses could possible infect humans and have surface proteins (H and N proteins) not previously seen by the human immune system.
In the last century there have been four main worldwide pandemics:
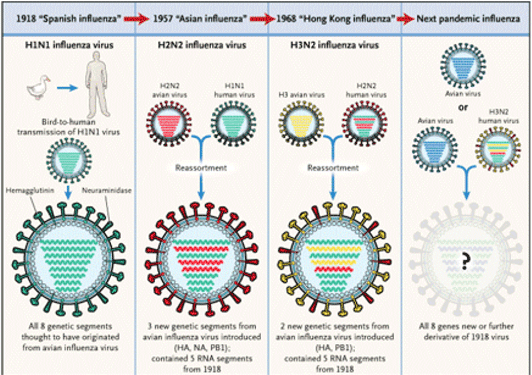
Source: New England Journal of Medicine, http://www.nejm.org/doi/full/10.1056/NEJMp058281
Are we prepared?
This is a 15 minute talk at TED.com given by Laurie Garrett. This talk initially focuses on the potential threat from H5N1, so called "bird flu," but her remarks then turn to the threat from pandemic flu in general, and she has incisive observations about "preparedness" for pandemic influenza.
Influenza epidemics are influenced by several factors:
How a specific population is characterized by immunization status, age composition, and the number of people in the population at higher risks all affected the factors of an influenza epidemic.
The latency period of influenza, the infectious period, mechanisms of transmission, and virulence of the influenza strand are considered the pathogenic [insert rollover—disease causing agent = pathogen] traits which are also determinants.
Behavioral and lifestyle characteristics include population density, economic/political factors, and personal behaviors. These factors are more social depending on human interactions.
The environment and climate are the final factors that determine influenza infection. In the northern hemisphere "flu season" primarily takes place during the winter months (CDC, 2011). The influenza virus seems to flourish best in the cooler weather. Flu season can start in October and span into April (CDC, 2011). Below is a graph which depicts the frequency of visits to US outpatient facilities for influenza-like illness during the past several years. Note that the frequency generally begins climbing in the fall, peaks in mid-winter, and then declinces sharply. However, there is variability in the pattern, and the H1N1 pandemic that occurred in 2009-2010 was very unusually with a large spike in spring followed by a sharp decline that contiued throughout the winter.
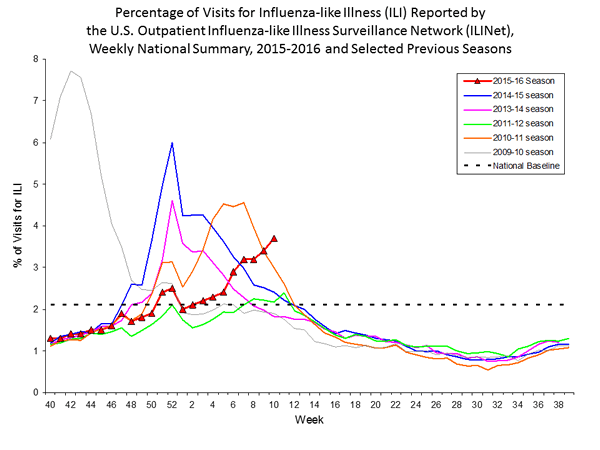
Source: http://www.cdc.gov/flu/weekly/summary.htm/
Measures can be taken to reduce one's personal risk and the risk of transmitting the flu virus. These include voluntary household isolation and quarantine, social distancing, widespread adoption of respiratory hygience and hand washing, and targeted use of antiviral medications. These are summarized in the table below.
|
Personal Habits |
Disinfection |
Social Distancing |
|---|---|---|
|
Practice appropriate handwashing or use of alcohol-based hand sanitizer before eating, drinking, touching the face or coming in contact with germs |
Utilize and wash eating utensils, dishes, and linens separately |
If infected, avoid hugging or other forms of intimate contact with individuals
|
|
Cover mouth & nose with tissue, arm or elbow when sneeze or cough |
Clean fomites and hard-surfaces with registered antimicrobial products in infected homes Antimicrobial products include: Lysol, Square, Burmishine Germicidal Solution, Zep Refresh II. A more comprehensive list can be found at this link Antimicrobial Products Registered for Use Against Influenza A Virus on Hard Surfaces |
Limit contact with infected individuals, vice versa |
|
Dispose of used tissues appropriately |
Wear a facemask if infected and have to be in close proximity with uninfected individuals Wear an N95 respirator if uninfected, but have contact with individuals infected with a pandemic strain |
|
"Based on an influenza-associated mortality rate of 0.5% (as has been estimated for New York City in the 1918–1919 pandemic), the magnitude of the predicted benefit of these interventions is a reduction from 49% to 27% in the proportion of the population who become ill in the first year of the pandemic, which would correspond to 16,000 fewer deaths in a city the size of Hong Kong (6.8 million people)" (Wu et al.: Reducing the impact of the next influenza pandemic using household-based public health interventions. Plos Medicine, 2006;3(9).
Research by Haber et al. (2007) supported the findings of Wu et al. and also showed that limited contact among infected individuals in long-term care facilities "decreased the rates of illness, hospitalization, and death for long-term care facility residents by >50% …. decreased the rates of hospitalization and death in the general population by up to 14% and 24% respectively"
|
The World Health Organization has developed a global surveillance program in which virus strains are collected by national flu centers around the world and samples of interest are sent to WHO's advanced laboratories for further assessment.
Every year, WHO suggests three virus strains (2 influenza A and 1 influenza B) to be included in each country's upcoming flu vaccine (FLU, 2011). Selection is based on circulation and ability to cause mass illness in the coming flu season. The chosen strains used to create the vaccine will provide protection against strains that are the same or related.
As indicated above, WHO only provides a recommendation for the upcoming flu vaccine and each country makes the final decision for their population. In the United States, the Food and Drug Administration (FDA) makes the final decision (FLU, 2011). The FDA has chosen A/California/7/2009 (H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus, and B/Brisbane/60/2008-like virus to be included in the 2011-2012 flu vaccine (CDC, 2011).
Two types of vaccines are manufactured for influenza:
Inactivated vaccine—consists of dead viruses that are given through injection. The three types of flu shots available are regular (>6 mo. of age), Fluzone High-Dose (>65 yrs. old) and intradermal (18-64 yrs. old) (CDC, 2011).
Attenuated vaccine—also known as live attenuated influenza vaccine (LAIV), is available as a nasal-spray (FluMist). As indicated by its name, the virus within the vaccine is weakened and will not cause the recipient to get the flu. Individuals under the age of 2 and over 49, pregnant, have a chronic medical condition, a weakened immune system or take medications that weaken their immune system should not get the vaccine (CDC, 2011).
Unless there is a contraindication, everyone 6 months of age or older should get vaccinated. However, vaccination is particularly important in certain groups. The following table from the CDC provides a list of those who should be most strongly encouraged to get vaccinated and also provides a list of those in whom vaccination is contraindicated.
Who Should Get Vaccinated
Who Should Not Get Vaccinated
In the past, emphasis has been placed on vaccinating individuals in high risk categories and healthcare providers during vaccine shortages first. In addition, sub-prioritizing is conducted at the discretion of state and local health officials and healthcare providers (CDC, 2011).
In the state of Massachusetts, high risk categories and their order of priority are (MA Department of Public Health, 2004):
In the case of a pandemic, prioritization is illustrated in the figure below from the CDC.
Title: Vaccination target groups, estimated populations, and tiers for severe, moderate and less severe pandemics as defined by the Pandemic Severity Index (PSI).
Persons in occupational groups not specifically targeted for vaccination in Moderate and Less Severe pandemics are targeted according to their age and health status in the general population.
; .
Source: http://www.flu.gov/images/reports/pi_vaccine_allocation_guidance.pdf
However, new information suggests that traditional prioritization of the elderly over healthy young adults and children during shortages of vaccine and pandemics should be reconsidered. Past research has revealed that children are high-transmitter groups (as cited in Miller et. al, 2008). This is most likely due to poor hygienic habits and minimal to no exercising of social distance when infected. Research findings have also suggested using children to create herd immunity as it is more effective to immunize a small group of children to protect the elderly than it is to immunize a very large group of the elderly (as cited in Miller et al, 2008; Radonovich, Bender, & Small, 2007). Consequently, because annual epidemics tend to hit school children first, there may be a rationale for vaccinating them first, rather than last. Overall, there is a greater potential to control the spread of influenza by vaccinating healthy young adults and children before the elderly. This is further illustrated by the following facts (Miller et. al, 2008; as cited in Miller et. al, 2008; Randonvich et. al, 2007):
In the state of Massachusetts, it is estimated that there will be a shortage of flu vaccine during the 2011-2012 flu season as a result of billion dollar budget cuts. Consequently, in the coming year state-supplied vaccines will be prioritized for children who are 6 months —18 years of age within identified cohorts and uninsured or underinsured adults who are at high-risk for complications if infected with the influenza virus (MASS, 2011).
Massachusetts recommends (MASS, 2011):
All licensed healthcare facilities are required to offer their employees free flu vaccinations according to state regulations within 105 CMR (MASS, 2011). To ensure yearly provision of state-supplied flu vaccine, all healthcare workers are required to report state-supplied vaccine usage to MDPH by the specified deadline (MASS, 2011).
Through the Vaccine Adverse Event Report System (VAERS) and the Vaccine Safety Datalink (VSD) Project, the CDC, FDA, and their partners are able to monitor the safety of the current flu vaccine by identifying adverse events and health complications that take place following vaccination (CDC, 2011). The data provided is also a means for: monitoring pattern changes in known adverse events, assessing the safety of groups that are at high-risk for complications, identifying individuals with a greater risk for certain adverse events, and assessing the safety and effectiveness of vaccine lots [insert rollover—batch of vaccine] (CDC, 2011).
These drugs are designed to treat infection and any associated complications of individuals infected with the influenza virus or serve as a form of prevention for non-infected individuals who are allergic to eggs, have developed Guillain-Barre Syndrome from exposure to the influenza vaccine or have a negative immune response to the vaccine. They consist of Amantadine (Flumadine), Rimantadine, Zanamivir (Relenza), and Oseltamivir (Tamiflu).
Surveillance in the United States and around the world is an important step in identifying possible influenza epidemics and classifying which influenza strains go into the yearly vaccines. There are 136 national influenza centers in over 100 countries worldwide that conduct year-round surveillance of influenza, and all information on trends and strains are reported to five World Health Organization Collaborating Centers for Reference and Research on Influenza in the United States, the United Kingdom, Australia, Japan and China.
In the United States, the Epidemiology and Prevention Branch in the Influenza Division of the CDC deals with analyzing influenza activity every year. Over 80 laboratories in the United States and abroad provide information on virologic surveillance, including the total number of respiratory specimens tested and the number positive for influenza types A and B each week (Center for Disease Control and Prevention, 2011). Hospitals and healthcare facilities also provide numerous influenza cases to the CDC, thereby providing up-to-date prevalence and incidence rates in all 50 states. Mortality and hospitalization surveillance track the number of influenza-related deaths and the number of patients admitted to hospitals with laboratory-confirmed influenza, respectively. Lastly, the surveillance system also tracks the geographic spread of influenza. All of this information is important in controlling influenza mutations, identifying strains for the yearly vaccinations and categorizing susceptible populations.
The figure below shows the percentage of visits to US outpatient facilities for influenza-like illness by month and shows how it varies during the year.

Source: http://www.cdc.gov/flu/weekly/summary.htm/
Syndromic surveillance uses indicators from individuals and populations before laboratory results and confirmed diagnoses to identify outbreaks and to monitor the health status of the community (Center for Disease Control and Prevention, 2011). This type of surveillance is important because it more up-to-date and often provides information quicker than a laboratory test can be completed.
Current weekly reports on influenza activity in the US can be found at http://www.cdc.gov/flu/weekly/, while worldwide reports may be accessed at http://www.who.int/influenza/gisrs_laboratory/flunet/en/. Google Flu Trends uses aggregated search data to estimate influenza activity around the world - http://www.google.org/flutrends/