Multiple Drug-Resistant Tuberculosis
MDR-TB and XDR-TB
Authors:
Camille Blackman
Rachel Browning
Dorie Kogut
Heather Young
Introduction
Tuberculosis (TB) is an infectious disease that can affect any organ in the body, although the vast majority of cases involve only the lungs. Transmission of the disease occurs from person to person via the airborne route. When an untreated individual with active TB coughs, droplet nuclei containing the TB bacillus are expelled into the air and can be inhaled by others in close proximity. Beginning in the late 1940s antibiotics were developed that were effective in curing TB. However, mutant strains of TB that were resistant to one or more of these antibiotics began to be identified as early as 1956. Since then, the evolution of antibiotic resistance among TB has been a growing problem that is now a major public health threat. Multiple drug resistant TB (MDR-TB) refers to strains of TB that are resistant to at least isoniazid and rifampin, two of the first-line antibiotics used in treatment. As the problem continued to grow, the term XDR-TB was coined for extensively drug resistant TB. XDR-TB is resistant to at least four of the first line anti-TB drugs, i.e., resistance to not only isoniazid and rifampicin, but also resistance to any of the fluoroquinolones and to at least one of three injectable second-line drugs (amikacin, capreomycin or kanamycin). MDR-TB and XDR-TB both take substantially longer to treat than ordinary (drug-susceptible) TB and require the use of second-line anti-TB drugs, which are more expensive and have more side-effects than the first-line drugs used for drug-susceptible TB. This module will focus on MDR-TB and XDR-TB.
|
Key Facts About TB from the World Health Organization (WHO)
|
- Tuberculosis (TB) is second only to HIV/AIDS as the greatest killer worldwide due to a single infectious agent.
- In 2012, 8.6 million people fell ill with TB and 1.3 million died from TB.
- Over 95% of TB deaths occur in low- and middle-income countries, and it is among the top three causes of death for women aged 15 to 44.
- In 2012, an estimated 530 000 children became ill with TB and 74 000 HIV-negative children died of TB.
- TB is a leading killer of people living with HIV causing one quarter of all deaths.
- Multi-drug resistant TB (MDR-TB) is present in virtually all countries surveyed.
- The estimated number of people falling ill with tuberculosis each year is declining, although very slowly, which means that the world is on track to achieve the Millennium Development Goal to reverse the spread of TB by 2015.
- The TB death rate dropped 45% between 1990 and 2012.
- An estimated 22 million lives saved through use of DOTS and the Stop TB Strategy recommended by WHO
About 450 000 people developed MDR-TB in the world in 2012. More than half of these cases were in India, China and the Russian Federation. It is estimated that about 9.6% of MDR-TB cases had XDR-TB.
|
Additional information on TB and how it spreads can be found at http://www.cdc.gov/tb/.
Learning Objectives
- Define and distinguish MDR-TB and XDR-TB.
- Explain the mechanisms by which resistance occurs.
- Describe the means of transmission.
- Describe the role of direct observation therapy (DOT) and its importance in preventing the spread of MDR-TB.
- Discuss potential consequences of not controlling the spread of MDR-TB and XDR-TB.
- Summarize the ongoing initiatives to control MDR-TB and XDR-TB on a global stage.
A Brief History of TB
The TB bacterium was identified in the late 1800's, and shortly after a TB test was created. However, it was not until the late 1940s that an effective antibiotic for treatment of TB was available.
Antibiotic Resistance and the Emergence of MDR-TB
Tuberculosis has existed for many centuries, but it was not until 1944 effective effective antibiotic therapy (streptomycin) became available. In 1950, scientist Renee Dubos predicted that that bacteria would eventually develop resistance to antibiotics through random mutations and natural selection. Before long, it was found that some TB patients had strains of TB that were resistant to treatment with streptomycin. Over the next two decades additional anti-tuberculosis drugs were introduced, including p-aminosalicyclic acid, isoniazid, and rifampin, but as their usage increased, so did the appearance of TB strains that were resistant to one or more drugs. In 1956 strains of TB that were resistant to streptomycin, para-aminosalicylic acid (PAS), and isoniazid (INH) were discovered in Great Britain and were eventually dubbed multi-drug resistant TB or MDR-TB. In subsequent years, when strains with resistance to an even greater number of antibiotics were discovered, the term "extensively drug resistant TB" (XDR-TB) was coined.
Random Mutation and Natural Selection
Characteristics that provide resistance to treatment are initially introduced by random mutations that occur. The video below provides a brief description of how random mutations and natural selection work together to introduce new strains of résistant bacteria.
The likelihood that a new mutant strain with some degree of resistance to treatment with a particular antibiotic will survive is dependent on several factors.
- First, the mutation may confer some degree of resistance which is an advantage, but the same trait may have other negative consequence, such as a slower growth rate. The overall effect will depend on the magnitude of these changes and also on environmental pressures. For example, a mutation conferring both antibiotic resistance and a slower growth rate result in a net disadvantage in the absence of that particular antibiotic. However, if the bacterium exists within and individual being treated with the antibiotic to which resistance has developed, then the net effect is a distinct advantage over the other TB which are sensitive to the antibiotic. Antibiotic resistance a selection pressure, because susceptible bacteria will be killed if treated with a sufficient dose over a sufficient amount of time, but resistant bacteria will survive and have less competition for nutrients and space.
- A second factor is the rigor of treatment. Some mutations provide partial, but incomplete resistance to a drug. If an individual harboring such a mutant form is treated with an appropriate dose of antibiotic according to an appropriate dosing schedule and for a sufficiently long period to time, then the mutant is unlikely to survive. However, with sporadic or incomplete treatment, then the mutant is likely to survive.
- Thirdly, if a mutant organism becomes resistant to one drug, but remains sensitive to others, then treatment with multiple drugs will greatly reduce the likelihood that the new mutant will survive. This provided the rationale for using triple antibiotic therapy. Antibiotic resistance to MDR-TB and XDR-TB is becoming increasingly common as many TB patients are developing multiple strains of different mutant bacteria (mixed infection). Multi-drug treatment therapy is essential in order to effectively control and cure patients with multiple strains of bacteria. Administration of a single drug often is ineffective and results in the development of antibiotic resistance. Multi-drug therapies containing three to four different drugs are effective because they minimize the overall risk of resistance by simultaneously eliminating different strains of susceptible bacteria (CDC, 1993). Nevertheless, some strains have acquired traits that provide them with resistance to multiple drugs (MDR-TB and XDR-TB), creating an enormously challenging clinical problem.
In view of these observations, it is not surprising that individuals who are most at risk of acquiring MDR-TB are those who:
- Do not complete standard TB therapy as prescribed
- Develop TB again after a previous TB infection
- Come from MDR-TB endemic areas
- Live in close proximity with MDR-TB carriers
Mechanisms of Resistance
Strains of Mycobacterium tuberculosis have undergone a number of mutations that enable them to "resist" treatment with a number of antibiotics. The activity below lists a number of anti-TB drugs and the mechanism by which one or more strains of TB have become resistant.
The video below is a 31 minute long lecture on mechanisms of antibiotic resistance.
Horizontal Gene Transfer
A bacterium that has acquired resistance to one or more antibiotics will transfer these traits to its offspring; this is referred to as vertical gene transfer (vertical evolution) which occurs when bacteria divide giving rise to two daughter cells by fission. However, resistance to antibiotics can also be spread through a process called horizontal gene transfer (HGT). HGT can occur via three transfer mechanisms:
- Conjugation- Direct cell to cell contact between two closely related bacteria, where plasmids (small DNA pieces encoded with the mutation) are transferred between cells.
- Transformation- Parts of DNA are taken up by the bacteria from the external environment. These DNA parts are the result of DNA death and lysis of other bacterium.
- Transduction- Bacteriophages (bacteria-specific viruses) transfer DNA between two closely related bacteria.
Diagnosis of TB and MDR-TB
Early diagnosis is key for MDR-TB for two reasons:
- Practitioners can ensure that the proper treatment is used on MDR-TB patients, avoiding medications where the patient is immune (Nathanson, Nunn, Uplekar, Floyd, Jaramillo, Lonnroth, Raviglione, 2010). In 2007, approximately 15% of MDR-TB cases were predicted to actually be XDR-TB strains (Shah, Wright, Bai, Barrera, Boulahbal, et al., 2007). As MDR-TB cases are resistant to isoniazid and rifampicin (first-line TB drugs), XDR-TB strains are resistant to first-line drugs, as well as fluoroquinolones and at least one of the second-line injectable drugs.
- The sooner proper treatment commences, the sooner MDR-TB will be contained in the community.
In order to diagnose MDR-TB, drug-resistance testing should be conducted to determine if the TB strain is resistant to any drugs other than rifampicin and isoniazid (Nathanson et al., 2010). This is can be done a few different ways. We will begin with the most efficient, which are the rapid molecular tests.
Rapid Molecular Tests
Rapid molecular tests are now available to detect resistant strains. These tests are not universally available, but WHO strongly recommends that these be used in the first-line of diagnosis when MDR-TB is suspected or when working with HIV-infected patients. the Xpert MTB/RIF diagnostic test produces results within the same day so patients can begin treatment quickly. Implementation of this test and other technologies are slowly being integrated into practice, but will greatly impact MDR-TB case management. Additional Information is available at http://www.who.int/tb/features_archive/factsheet_xpert.pdf (Nathanson et al., 2010).
Other rapid molecular tests in the field include NRA, MODS, Genotype MTBDR, and Genotype MTBDRplus. These diagnostic tests can take 2-29 days to provide results (Bwanga, Hoffner, Haile, and Joloba, 2009). Additional information about the sensitivity and specificity of these tools can be found at: https://learn.bu.edu/courses/1/13fallsphph709_a1/groups/_25651_1//_1765668_1/Direct%20Susceptibility%20Testing%20of%20MDR-TB.pdf.
Conventional Drug-Susceptibility Tests
Conventional drug-susceptibility tests can be conducted using solid or liquid culture from the patient. Diagnosis using this route can take more time since it typically cannot be conducted on-site. The common turn around time using this method is about two months (Bwanga et al., 2009). The use of these tests are crucial among patients at risk of MDR-TB (Nathanson et al., 2010). According to the Center for Disease Control (CDC), these groups include:
- Prior TB disease treatment;
- Contact with a patient with known anti-TB drug resistance;
- Demonstrated resistance to first-line anti-TB drugs; or
- Positive cultures after more than 3 months of treatment.
If possible, it is best to conduct both molecular and conventional drug-susceptibility testing. Although molecular testing can provide a quick diagnosis for many disease mutations, others still remain unknown. Therefore, it is important to conduct both tests when working with patients suspected of contracting MDR-TB.
Prevention
MDR-TB can occur in two ways:through incorrect treatment of TB (via misdiagnosis or low treatment adherence) or when MDR-TB is present in the community and the airborne disease spreads to others. The best practice for prevention is to ensure that TB patients complete their treatment course (ALA, 2013).
To prevent the spread of MDR-TB, CDC recommends inhibiting exposure to MDR-TB cases in crowded areas, such as hospitals, prisons and homeless shelters (CDC, 2012).
Surveillance of MDR-TB in the United States and Worldwide In 1993, the CDC expanded its TB surveillance system to include MDR-TB cases. Additional information about the National TB Surveillance System can be found at: http://healthindicators.gov/Resources/DataSources/NTBSS_120/Profile.
WHO also monitors the incidence of drug resistance in TB. Most recently, they began reporting on the progress of treating MDR-TB. WHO reports and publications can be found at http://www.who.int/tb/publications/mdr_surveillance/en/.
Treatment
MDR-TB occurs when TB is not treated correctly or the patient does not adhere to their medication regimen. The best practice for prevention is to ensure that TB patients complete their treatment course (ALA, 2013). Once MDR-TB is present, it must be treated to prevent further health issues for the patient and to prevent the spread of MDR-TB to others in the community. The main obstacle for MDR-TB treatment is medication adherence. Treatment may involve taking multiple medications for up to two years that include adverse side effects such as headache, upset stomach, dizziness and skin rash (North Dakota Department of Health, 2013).
Direct Observation Therapy (DOT)
DOT is a process where medication adherence is observed. Practitioners use DOT on a wide range of TB cases, from latent TB in children to active MDR-TB patients. Once the treatment course is prescribed, a trained healthcare worker gives prescribed TB drugs to the patient and then observe the patient swallow the drugs or receive the injection. Healthcare workers may also check for side effects and ensure the patient is healthy. Therapy may occur anywhere from just the first two months of therapy to the full course of treatment. Additional Information on DOT can be found at: http://www.health.state.mn.us/divs/idepc/diseases/tb/lph/dot.pdf.
Although DOT ensures adherence, it is labor intensive and time consuming for both the patient and the health system. Many organizations have begun researching other ways to conduct DOT, such as video monitoring, time-released pill bottles and even ingestible sensors (Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, et al., 2013).
The video below provides a brief report on the use of DOT and fingerprinting to treat and control TB in India.
Surgical Therapy
According to a literature review funded by the National Institutes of Health (NIH) and the Atlanta Clinical and Translational Science Institute, no randomized controlled trials using artificial pneumothorax surgery in combination with antibiotic treatment on MDR-TB have been conducted, but based on the trends in the research conducted thus far, it is reasonable to consider surgical resection early in the course of treatment.
Surgical therapy may be an option for MDR-TB patients that meet the following criteria:
- Such extensive drug resistance that there is a high likelihood of treatment failure or relapse
- Localized disease amenable to resection 3) Sufficient drug activity to reduce remaining mycobacterial burden enough to allow bronchial stump healing.
Other considerations:
- Surgery for tuberculosis should only be performed at specialized surgical facilities or experienced thoracic surgeons.
- No research has been conducted on the efficacy of surgery specifically on immuno-compromized patients (Kempker, R., Vashakadze., Solomonia, N., Dzidzikashvili, N., Blumberg, H., 2013).
The Future of MDR-TB & XDR-TB
The result of incorrectly treating for TB or not completing an antibiotic treatment regimen resulted in MDR-TB. To control MDR-TB, second-line drugs were developed. However, in 1993, TB strains further resistant to some of those second-line treatments emerged across the world due to improperly treated MDR-TB. These strains are known as XDR-TB and are a threat to the future of global TB control.
|
|
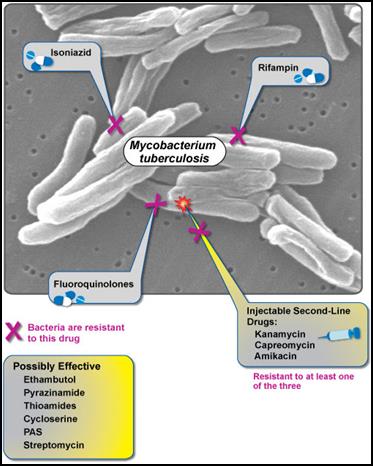
Figure 1: XDR-TB is resistant to fluoroquinolones, isoniazid, rifampin, and at least one second-line injectable drug. Currently, no effective cure is known for XDR-TB. (NIAID, 2007).
According to USAID, XDR-TB is defined as a case of TB resistant to at least isoniazid and rifampicin (among the first-line anti-TB drugs), as well as resistant to any fluoroquinolone treatment and at least one second-line injectable anti-TB drug (amikacin, capreomycin, or kanamycin) (USAID, 2009).
XDR-TB evolved to resist the antibiotics because patients stopped taking their pills too early--once they felt better but before their treatment cycle was complete--which failed to kill the MDR-TB in their bodies. By not completing the regime, those patients spared the toughest strains--TB that had mutated to become even less susceptible to the drugs. This is not uncommon in poor countries where patients and clinics are unable to afford any, enough, and the right combinations of the high-quality drugs necessary to defeat the resilient strains (Shufro, 2007). Symptoms and methods of transmission are the same as TB and MDR-TB, but the more resistant the TB, the more difficult the bacteria is to control, the more fatal the disease, and the more dire the prospects. In March, 2013, WHO estimated that of the approximate 310,000 cases of MDR-TB in the world, about 9% were XDR-TB and located in 84 different countries (ALA, 2013).
|
XDR-TB as a Global Problem
While the number of XDR-TB cases among foreign-born people between 1993 and 2006 was similar, the percentage of foreign-born cases increased from 39% to 76%, whereas the US cases declined. At least 67% of all XDR-TB cases in 2011 were disproportionate among foreign-born people, according to the American Lung Association (ALA, 2013 ). Although more people outside the US are afflicted with XDR-TB, the disease does not discriminate and may easily travel anywhere around the globe, deeming XDR-TB a global issue.
|
|
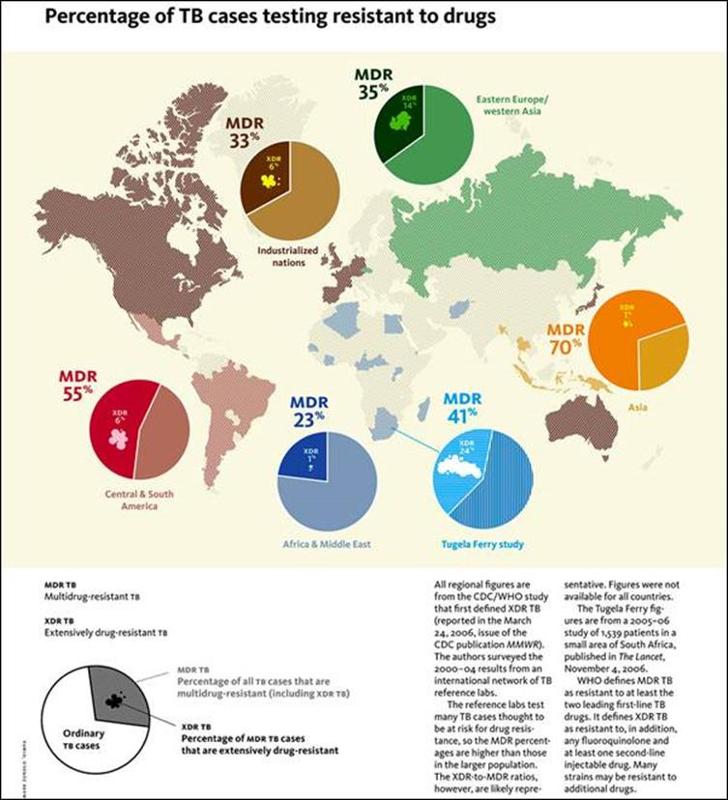
Figure 2: The map on the left uses data from the CDC/WHO (2006) to show percentages of areas around the world most afflicted with TB, including drug-resistant TB. The pie charts indicate the proportion of TB cases that are MDR-TB and the figures within the MDR-TB pie slices depict the proportion of XDR-TB. Although Asia has the highest percentage of MDR-TB, Eastern Europe/Western Asia has the highest percentage of XDR-TB. The presence of XDR-TB around the world demonstrates the need to quickly suppress TB cases and, in particular, prevent further spread of XDR-TB (Yale Alumni Magazine, 2010).
|
Drugs used to treat MDR-TB in comparison to TB are expensive, the routine treatment complex, and the side-effects more harmful, therefore, the assumption is that the discovery and implementation of effective third-line drugs used to treat XDR-TB will require exponentially more money, time, and effort resulting in drugs that require longer treatment periods and have even more deleterious side-effects. No new TB drugs have been developed over the past 40 years, explains Gerald Friedland, a Yale medical school professor and director of Yale's AIDS program as well as a lead scientist for a TB and HIV co-infection study in South Africa : "'doctors whose patients face resistant TB can't turn to "third-line" drugs: none exists'" (Shufro, 2007). In a CDC pod cast titled "Emergence of Extensively Drug Resistant Tuberculosis," Dr. Peter Cegielski, team leader for drug-resistant TB in the Division of Tuberculosis Elimination at CDC, is asked questions about XDR-TB. He stated that XDR-TB cannot be treated efficiently because efficient treatment calls for at least four effective drugs, which are unavailable for the magnitude of resistance posed by XDR-TB. To add, there are currently no existing third-line drugs powerful enough to cure XDR-TB (CDC, 2008). The third-line drugs that are available include: clofazimine, linezolid, amoxicillin plus clavulanate, imipenem plus cilastatin, and clarithromycin, but their efficacy is unclear and roles undefined (Zumla, Nahid, & Cole, 2013). In addition, "the cost of second-line drugs had always been so high that middle- and lower-income countries couldn't afford them," (CDC, 2008), implying that third-line drugs are also too costly for most afflicted with MDR- and XDR-TB.
Monitoring programs, like DOT, and record-keeping databases, like NTBSS, while effective in treating and controlling for TB, are not as effective for MDR-TB and XDR-TB because not all cases of resistant TB are diagnosed correctly. As Dr. Cegielski states, "not every lab tests every isolate for all of the second-line drugs"(CDC, 2008); resistance may not be just due to one drug, but a combination of drugs (CDC, 2008); and testing may take weeks to months to confirm resistance. According to scientists studying the effectiveness of innovative drug-resistant-TB technology, "the burden of XDR-TB is increasing due to inadequate monitoring, lack of proper diagnosis, and treatment" (Singh, Maurya, Kant, Umrao, Kushwaha, Nag, & Dhole, 2013). The technique of diagnosing resistant TB via sputum sample, used in poor countries, is antiquated and time-consuming (Shufro, 2007). To prevent the further spread of XDR-TB, locating and confirming cases early and quickly, allocating necessary effective third-line drugs, and monitoring treatment regimes of those with the disease before individuals transmit the bacteria are crucial requirements for decreasing the incidence and prevalence of XDR-TB across the globe.
Without effective, accessible, and affordable third-line drugs and monitoring programs to ensure proper diagnosis and treatment compliance, XDR-TB will continue to be a global threat affecting a myriad of people across the globe, and particularly in developing and impoverished nations.
HIV and XDR-TB, a Fatal Combination
Immuno-compromised people are more likely to contract TB, and the combination of HIV and MDR- or XDR-TB can be fatal. With drugs prolonging the lives of HIV-positive individuals and with no current cure for HIV, the incidence of XDR-TB increases and poses a serious problem. While 75% of healthy people with MDR-TB recover, the death rate for HIV-infected people who have also contracted MDR-TB is 40-60% (Shufro, 2007).
|
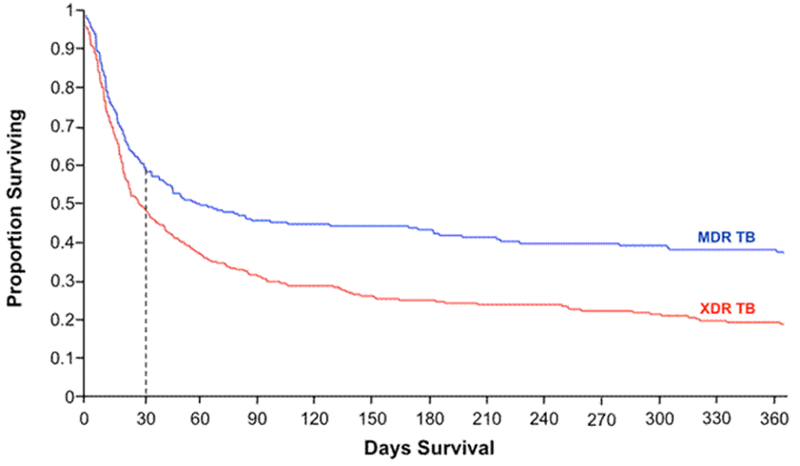
Figure 3: The Kaplan-Meier survival plot from a retrospective observational study of 272 MDR and 384 XDR-TB cases co-infected with HIV in South Africa shows the survival disparity between the two groups. The graph indicates that those with MDR-TB and HIV have a 40% chance of survival, whereas those with XDR-TB and HIV have a 20% chance, supporting the claim that those with XDR-TB have a higher relative risk of early mortality than those with MDR-TB (HIV Web Study, 2010).
|
|
A study conducted by Yale University between 2003 and 2007 on HIV and drug-resistant TB in rural South Africa defined the growing global problem with XDR-TB. Of the 53 patients with XDR-TB, all of them were also confirmed or suspected HIV cases. 52 of those patients died with an average survival rate of less than a month after their sputum was taken for testing: "'what was repeated over and over again was the mortality rate: 98 percent...people are waking up. They are realizing that TB is a worldwide catastrophe,'" proclaimed Friedland, one of the lead scientists (Shufro, 2007).
Again, the problem of affordable and accessible drugs persists. Yale discovered that South African doctors only had two or three expensive second-line drugs to treat MDR-TB, however, they were less effective. When the drugs did not work, patients died (Shufro, 2007).
"'This is an issue of grave worldwide importance...MDR and XDR [TB] carry the danger of blunting or reversing the success of TB programs and the roll-out of anti-retroviral [sic] therapies for HIV where they are desperately needed in resource limited settings,'" said Friedland. He calls for urgency in intervening to treat and prevent drug-resistant TB, especially now that MDR- and XDR-TB are more prevalent than previous thought and that XDR-TB and HIV co-infections are associated with high mortality (ScienceDaily, 2006).
Can XDR-TB be contained? Can epidemiologists fully predict its course? Or will it become, as Friedland called a "'slow tsunami,'" like AIDS, "'a vague and yet very palpable feeling of dread that something very bad was happening'" (Shufro, 2007).
Are There Any Solutions?
In the CDC pod cast, Dr. Cegielski is asked to share his thoughts on a solution to the influx of XDR-TB cases: "'we need stronger labs in countries that are most infected [sic] by drug-resistant TB. And we need new drugs. But those are years in the future'"(CDC, 2008). TB continues to grow resistant to the second-line drugs faster than scientists can find new, effective third-line drugs to treat XDR-TB. Research must be focused on increasing the effectiveness of overseas labs in diagnosis and treatment of MDR- and XDR-TB, as well as focused on discovering stronger third-line drugs. 40 years is too long to wait for a new treatment to a quickly spreading and potentially fatal disease.
A 2013 study conducted in India, the country with the highest rates of TB and second highest rates of MDR-TB after China, according to WHO (National Academy of Sciences, 2012), tested the efficacy of a drug resistance testing device, GenoType® MTBDRsl assay in determining MDR- and XDR-TB cases. The scientists found that the GenoType ® MTBDRsl assay produced accurate second-line drug resistance diagnoses within 5 hours, as opposed to 1-2 months, which provided more information on the frequency of mutation trends responsible for XDR-TB resistance. Rapid diagnosis and identification of XDR-TB will aid in faster implementation of proper control techniques, such as isolation and country-specific TB action programs, to prevent the further spread of the disease.
|
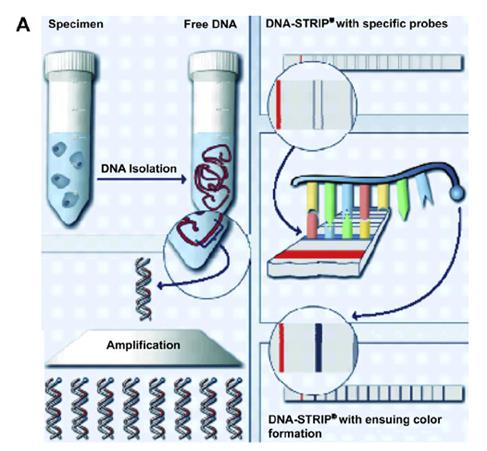
Figure 4: For XDR-TB screening, the DNA strip indicates whether the specimen contains TB resistant to fluoroquinolones, aminoglycosides/cyclic peptides, and/or ethambutol, evident via a dark line on the specific strip marker. The process is as follows:
1--TB DNA is extracted from the sputum sample 2--DNA is amplified via PCR
3--DNA is applied to strip with specific probes 4--DNA is detected on strip using reverse hybridization and enzymatic color reaction, resulting in a dark line when detection is positive. (Schaaf, 2009).
|
|
Continued efforts on educating the world about the importance of treatment compliance and the basics on TB may continue to help reduce the incidence of MDR-TB with hopes that XDR-TB, too, will be kept at bay.
References
- American Lung Association (ALA) (2013). Extensively Drug-Resistant Tuberculosis (XDR TB) Fact Sheet. Retrieved from http://www.lung.org/lung-disease/tuberculosis/factsheets/extensively-drug-resistant.html American Lung Association (ALA) (2013). Multiple Drug-Resistant Tuberculosis (MDR-TBMDR-TB) Fact Sheet. Retrieved from: http://www.lung.org/lung-disease/tuberculosis/factsheets/multidrug-resistant.html
- Belknap R., Weis S., Brookens A., Au-Yeung K.Y., Moon G., et al. (2013). Feasibility of an Ingestible Sensor-Based System for Monitoring Adherence to Tuberculosis Therapy. PLoS ONE 8(1): e53373. doi:10.1371/journal.pone.0053373
- Bwanga, F., Hoffner, S., Haile, M., Joloba, M. (2009). Direct Susceptibility Testing for a Multi Drug Resistant Tuberculosis: A Meta-Analysis. (2009). BioMed Central Infectious Diseases (9:67). doi:10.1186/1471-2334-9-67 Centers for Disease Control and Prevention (CDC). 2008. Emergence of Extensively Drug Resistant Tuberculosis. http://www2c.cdc.gov/podcasts/player.asp?f=4733
- Centers for Disease Control and Prevention (CDC): Division of Tuberculosis Elimination (2012). Tuberculosis: Drug Resistant TB. Retrieved from: http://www.cdc.gov/tb/topic/drtb/default.html Center for Disease Control (CDC) (1993). Initial Therapy for Tuberculosis in the Era of Multidrug Resistance -- Recommendations of the Advisory Council for the Elimination of Tuberculosis. Retrieved from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00020964.htm
- HIV Web Study. (2010). [Kaplain-Meier survival plot among MDR-TB and XDR-TB patients co-infected with HIV, 2005 to 2007]. HIV Coinfection in Multidrug- and Extensively Drug-resistant Tuberculosis Results in High Early Mortality. Retrieved from http://depts.washington.edu/ghivaids/reslimited/case6/discussion.html Kempker, R., Vashakidze, S., Solomonia, N., Dzidzikashvili, N., Blumberg, H. (2012). Grand Round Calling the Surgeon: The Role of Surgery in the Treatment of Drug-Resistant Tuberculosis. The Lancet, 12(2): 157-166. Doi:10.1016/S1473-3099(11)70244-4.
- Nathanson, E., Nunn, P., Uplekar, M., Floyd, K., Jaramillo, E., Lonnroth, K., ... Raviglione, M. (2010). MDR Tuberculosis--Critical Steps for Prevention and Control. The New England Journal of Medicine, (363), 1050-1058. doi:10.1056/NEJMra0908076. National Academy of Sciences. Facing the Reality of Drug-Resistant Tuberculosis in India. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK100386/
- National Institute of Allergy and Infectious Diseases (NIAID). (2007). [Graphic showing XDR-TB]. Extensively Drug-Resistant Tuberculosis (XDR-TB). Retrieved from http://www.niaid.nih.gov/topics/tuberculosis/understanding/whatistb/visualtour/pages/xdr-tb.aspx
- Schaaf, H., Moll, A., Dheda, K. (2009). [Graphic showing the process of testing for resistance using DNA strips]. MDR- and XDR-TB in Africa and South America. Retrieved from http://dc269.4shared.com/doc/Owot0-dG/preview.html
- ScienceDaily (2006). HIV/AIDS Linked To Extensively Drug Resistant TB. http://www.sciencedaily.com/releases/2006/11/061113170820.htm
- Second Line Anti-TB Medications. North Dakota Department of Health, Web. 14 October 2013. <http://www.ndhealth.gov/disease/tb/Documents/Second%20line%20TB%20drugs.pdf >
- Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, Drobniewski F, Gilpin C, Havelková M, Lepe R, Lumb R, Metchock B, Portaels F, Rodrigues MF, Rüsch-Gerdes S, Van Deun A, Vincent V, Laserson K, Wells C, Cegielski JP. (2007). Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis.13(3):380-7.
- Shufro, C. (2007). Tracking the Reaper: How a Handful of Doctors Found One of the Deadliest Kinds of TB in the World . Yale Alumni Magazine. http://archives.yalealumnimagazine.com/issues/2007_07/tb.html
- Singh, A.K., Maurya, A.K., Kant, S., Umrao, J., Kushwaha, R.A., Nag, V.L., & Dhole, T.N. (2013). Rapid detection of drug resistance and mutational patterns of extensively drug-resistant strains by a novel GenoType® MTBDRsl assay.



