
Overview of the 1999 Food Code
Primary Authors:
Diane Bernazzani, Massachusetts Department of Public Health, Bureau of Environmental Health - Food Protection Program
Raymond Duffill, U.S. Department of Health and Human Services, Food and Drug Administration
Liz Faye, Boston University School of Public Health


DISCLAIMER:
This training module is brought to you by the Local Public Health Institute of Massachusetts (the Institute) and by the New England Alliance for Public Health Workforce Development (Alliance). The Institute is a Massachusetts Department of Public Health funded entity created to strengthen local public health through training and education. It is managed by the Boston University School of Public Health's Office of Public Health Practice and is supported in part by the Center for Disease Control and Prevention (CDC)'s Grant/Cooperative Agreement Number 5U90TP116997-10, Public Health Preparedness and Response for Bioterrorism and the Alliance, funded by the Health Resources and Services Administration (HRSA) from 2000 - 2014. The contents of this module are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the HRSA. The authors of this module have no financial interests or relationships to disclose.
The U. S. Food and Drug Administration (FDA) publishes the Food Code, a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment of the industry (restaurants and grocery stores and institutions such as nursing homes). Local, state, tribal, and federal regulators use the FDA Food Code as a model to develop or update their own food safety rules and to be consistent with national food regulatory policy.
The Association of Food and Drug Officials (AFDO) reported in June 2005, that 48 of 56 States and territories have adopted food codes patterned after one of the five versions of the Food Code (Code), beginning with the 1993 edition. As of October 1, 2000, the Massachusetts Department of Public Health (MDPH) adopted and incorporated by reference the federal 1999 Code into the State Sanitary Code Chapter X: Minimum Sanitation Standards for Food Establishments 105 CMR 590.000 (590). However, certain provisions of the 1999 Code were specifically stricken or modified for conformance with State statute or laws.
The adoption of the federal 1999 Code is the core of the regulation, so it is very important to understand the structure of the Code and how to read it. This module will provide a brief overview of the 1999 Code including its structure and conventions, as well as the underlying food safety principles built into its framework. Food safety inspectors charged with enforcement of 590 should be familiar with the content presented in this module.
Participants of this module should be equipped with a copy of the 1999 Food Code.
Adoption of the FDA Food Code (Code) at the state, local and tribal level has been an essential component in the effort to promote greater consistency of food safety practices. However, it was clear that regulatory programs that administer the Food Code would also require a set of widely recognized standards. To meet this need FDA has developed, with broad stakeholder input, the "Voluntary National Retail Food Regulatory Program Standards" (Program Standards) to identify what constitutes a highly effective and responsive retail food regulatory program.
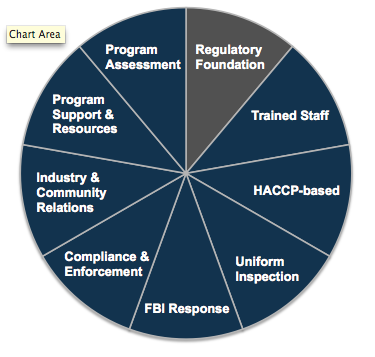
Regulatory Foundation is only one part of a comprehensive retail food protection program. The pie chart below depicts the eight additional components of "FDA's Recommended Retail Food Regulatory Program Standards."
The Code incorporates five key public health interventions to protect the consumer from foodborne illness which were introduced with the 1993 Code. Not surprisingly, they have been debated. Below are the five interventions.
Demonstration of Knowledge (2-102.11)
During inspections or upon request, the manager or person-in-charge (PIC) should be able to demonstrate knowledge of foodborne disease prevention, Hazard Analysis Critical Control Points principles, and the requirements of the Code. This includes complying with the Code, being a certified food protection manager, and being able to respond correctly to the inspector's questions as they relate to specific food operations.
Areas of knowledge include understanding the following:
Employee Health (2-201)
It is the responsibility of the person in charge (PIC) to require reporting by food employees information about their health and activities that may relate to the transmission of foodborne disease. Information should be reported in a manner that the PIC can prevent the transmission of foodborne disease, including the date of onset of jaundice or certain illnesses such as E. coli O157:H7, Shigella spp., Salmonella Typhi, and Hepatitis A.
Time/Temperature (3-401.11)
Raw animal foods including eggs, fish, meat, and poultry should be cooked in such a way as to heat all parts of the food to a given temperature for a given time, which will vary depending on the type of food being cooked. Examples of specific temperatures and times include:
Hands as a Vehicle of Contamination (3-301.11)
Procedure should be in place to limit hand contact as a potential vehicle of contamination. Food employees should wash their hands using proper technique, which is specified in §2-301.12. Food employees are not to handle exposed, ready-to-eat food with their bare hands, and should use utensils such as deli tissue, spatulas, tongs, single-use gloves, or dispensing equipment. Bare hand and arm contact with exposed food that is NOT in ready-to-eat form should be minimized.
Please refer to subpart 2-301 to answer the following questions.

Consumer Advisory (3-603.11)
This section is designed to provide the options available to food establishments when advising consumers of the inreased risk of foodborne illness upon consumption of raw or undercooked foods that are animal-derived. The image below illustrates the consumer advisory notice found on food menus.

Two major components to satisfactory compliance include disclosure and reminder.
Disclosure
Reminder
The Code also incorporates in its provisions the means to control the risk factors most frequently identified by the Centers for Disease Control as contributing factors in reported foodborne illness outbreaks.
The Code is a reference document for regulatory agencies responsible for overseeing food safety in retail outlets such as restaurants and grocery stores and institutions such as nursing homes and child care centers. It is neither federal law nor federal regulation and is not preemptive, but may be adopted and used by agencies at all levels of government that have responsibility for managing food safety risks at retail. The Code provides public health and regulatory agencies with practical science-based advice and manageable, enforceable provisions for mitigating risk factors known to contribute to foodborne disease. The Massachusetts Department of Public Health adopted by reference the federal 1999 Code into 590 to ensure that MA regulation will always conform to the federal standard.
Below is a basic organizational outline of the Code.
This portion of the Code provides background on the nature and extent of foodborne illness, information about the development of the code, and modifications and improvements from the previous edition. Section 8 of the preface also provides information on structure, nomenclature, and methodolody to help assist the user in navigating the various components of the Food Code.
Part 1-2 lists and defines terms that apply in the interpretation and application of the Code. Small capital letters are used wherever any word form of a defined term appears within the Code. The word "law" is an all encompassing term in the Code vs. specific to statutes. Its application may relate to plumbing codes or other local or state regulations impacting a food code. As an example, the words underlined in red in the image below, "PLUMBING SYSTEM", "LAW", "PLUMBING FIXTURE" and EASILY CLEANABLE" are all defined in Chapter 1. The term "LAW" means applicable local, state, and federal statutes, regulations, and ordinances (e.g. MA Plumbing Code).


The major food safety subject areas include personnel, food, equipment/facilities/supplies, as well as compliance and enforcement. Note that personnel was moved up to Chapter 2 because it is often people and personnel practices that cause foodborne disease. "People" Vs. "Food" (as of the 1993 Code) switched sequence as compared to previous codes. That is, "Food" used to be first after definitions. The reason for the shift was to emphasize the importance of the human element and role of fecal/oral contamination.
Chapter 8 deals with compliance and enforcement of the Code. Regulatory authorities can impose additional requirements in addition to the requirements contained in the Code if necessary to protect against public health hazards or nuisances. Variances that waive or modify the requirements of the Code can also be granted by a regulatory authority if such variance does not result in a health hazard or nuisance in the opinion of the regulatory authority.
The table below provides a breakdown of content by chapter.
|
Food Code Chapter |
Content |
|
2 - Management and Personnel |
Supervision; employee health; personal cleanliness; hygienic practices |
|
3 - Food |
Characteristics of food; food sources; protection from contamination after receiving; limitation and destruction of organisms of public health concern; food identity an labeling; handling contaminated food; and special requirements for highly susceptible populations |
|
4 - Equipment, Utensils, and Linens |
Materials for construction and repair; design and construction; numbers and capacity; location and installation of equipment; cleaning and sanitization of equipment and utensils; laundering; and protection of clean items |
|
5 - Water, Plumbing, and Waste |
Water; plumbing system; mobile water tank and mobile food establishment water tank; sewage, other liquid waste, and rainwater; refuse, recyclables, and returnables |
|
6 - Physical Facilities |
Materials for construction and repair; design, construction, and installation; numbers and capacity; location and placement; maintenance and operation |
|
7 - Poisonous or Toxic Materials |
Labeling and identification; operational supplies and applications; stock and retail sale |
|
8 - Enforcement and Compliance |
Code applicability; plan submission and approval; permit to operate; inspection and correction of violations; prevention of foodborne disease transmission by employees |
There are seven annexes to the 1999 Code. They were not adopted by 590, but still remain a great resource.
While the Code contains a lot of useful information and resources regarding food safety and the prevention of foodborne disease transmission, it is important to understand how the Code is organized and how it should be read.
The structural nomenclature of the Food Code takes the following order:

If section numbers end in .#0 or .#00, they are considered for information only and are non debitable provisions, so they are not recorded on inspections as violations because they are not written as violatable provisions.

Equipment that is certified by an accredited certification program is a method of satisfying the equipment requirements (deemed status). The equipment requirements are satisfied if the equipment criteria in §4-205.10 is met. If the criteria is not met, then the equipment must be evaluated on the spot to determine acceptability or deficiencies.
NOTE: Chapter 8 has many provisions that are not debitable, for example, §8-202.10 requires the Regulatory Authority to hold confidential, trade secrets.
The Code is organized in a "logical sense" and requires the user to think about what requirement they want to find. Basing provisions on principle rather than subject requires cross referencing but results in fewer words. The Code would be much larger if it addressed all requirements for refrigerators, then for sinks, then for ventilation systems, etc. As a result, extensive cross-referencing is used to avoid repetition.
There is a list of Parts at the beginning of each Chapter.
Example #1:
As illustrated below, the structure and flow of the "Parts" section found at the beginning of Chapter 3 of the Code follows a logical sequence - source, receiving, protection after receipt, contaminated food, addressing susceptible populations.

Example #2:
Illustrated below, the sequence of sections found in Chapter 4 is also logical starting first with materials needed for construction and repair, followed by design and construction, numbers and capacity required for equipment, utensils, and linen, and identifying the proper site for installation.

Keywords, such as 'Fingernails' in §2-302.11 and 'Jewelry' in §2-303.11, help to further narrow down the section of the code. This enables the reader to review a desired section of the Code more quickly. The photo album below illustrates how the keywords are listed differently in the hard copy pdf version of the Code versus the html version.
In response to the desire to allow discretion when encountering a violation, there is the convention of designating certain code provisions as non critical rather than critical or with the Swing designation permitting it to be either critical or non critical depending on the situation.
Requirements contained in the Code are presented as being in one of three categories of importance: critical; "swing" (i.e., those that may or may not be critical depending on the circumstances); and noncritical. An asterisk * after a tagline (which is the language immediately following a section number that introduces the subject of the section) indicates that all of the provisions within that section are critical unless otherwise indicated, as follows:
Any provisions that are "swing" items, are followed by the bold, superscripted letter S and any provisions that are noncritical are followed by the bold, superscripted letter N.

Any unmarked provisions within a section that has an asterisked tagline are critical. All provisions following a tagline that is not marked with an asterisk are noncritical.

Portions of some sections are written in italics. These provisions are not requirements, but are provided to convey relevant information about specific exceptions and alternative means for compliance. An italicized provision is not, itself, debitable. It allows some practice which, if not done according to the conditions stated, violates the general requirement.

Certain words used throughout the Code have very specific meanings and the language style adopted by the Code is based on Robert Marineau's writing format for regulations.
In the image below, the use of "shall" in paragraph A means that food MUST be obtained from approved sources. The language "may not" in paragraph B indicates that food prepared in a private home is PROHIBITED from being used or offered for human consumption in a food establishment. Finally, in paragraph C, "may" indicates that fish that are intended to be eaten raw are PERMITTED to be offered for sale or service under conditions further specified in §3-402.11 and §3-402.12.

Cross referencing is prevalent throughout the code. To know where certain violations should be debited, the context and language used will guide you.
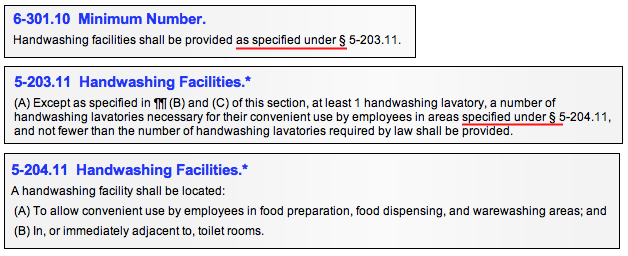
A code section preceded by the phrase "as specified in" refers to another section of the code or a document outside of the code for additional information to help you determine whether the item is in or out of compliance.
A provision that follows a statement such as "as specified in…" becomes an integral part of the Section. "In" is used (as opposed to "under") because the document being referenced is outside the Code (and unto itself not debitable) and therefore needs to be brought into the requirement. For instance, a reference may be made to a local plumbing code and plumbing must meet the specifications of that code.
Common examples of documents being referenced include "law" and the Code of Federal Regulations (CFR) as illustrated below.

"Under" indicates where a violation is properly recorded against the Code provision that follows "as specified under". A provision that follows a statement such as "as specified under" is referenced for information or in order to appropriately debit the provision. You must read the phrase in the context of the entire provision to make that determination. See examples below.

Hazard Analysis & Critical Control Points (HACCP) is a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product. As such, HACCP is a common sense approach to assuring food safety from harvest to consumption. Preventing problems from occurring is the primary goal underlying any HACCP system.
The Code includes HACCP principles.
A flow diagram that delineates the steps in the process from receipt to sale or service forms the foundation for applying the seven principles. The flow of food in a retail or food service establishment is the path that food follows from receiving through service or sale to the consumer. Most food items produced in a retail or food service establishment can be categorized into one of three preparation processes based on the number of times the food passes through the temperature danger zone between 41°F to 135°F. Below illustrates three general process flows.
There are basic sanitation requirements considered prerequisite to establishing a HACCP program, and is one of the first steps in developing a food safety management system. See Managing Food Safety: A Manual for the Voluntary Use of HACCP Principles for Operators of Food Service and Retail Establishments for more information. The purpose of developing prerequisite programs is to implement a strong foundation of procedures that address the basic operational and sanitation conditions within a food establishment. When such programs are in place, greater emphasis can be placed on the hazards associated with the food and its preparation.
Prerequisite programs include:
Such programs should be in place to control the contamination of food, control bacterial growth, and maintain equipment.
In developing a food safety management system, it is important for a food establishment to identify food safety hazards that exist in the flow of food from receiving to serving or sale. Once food safety hazards have been identified, it is possible to determine and implement control measures in order to achieve active managerial control.
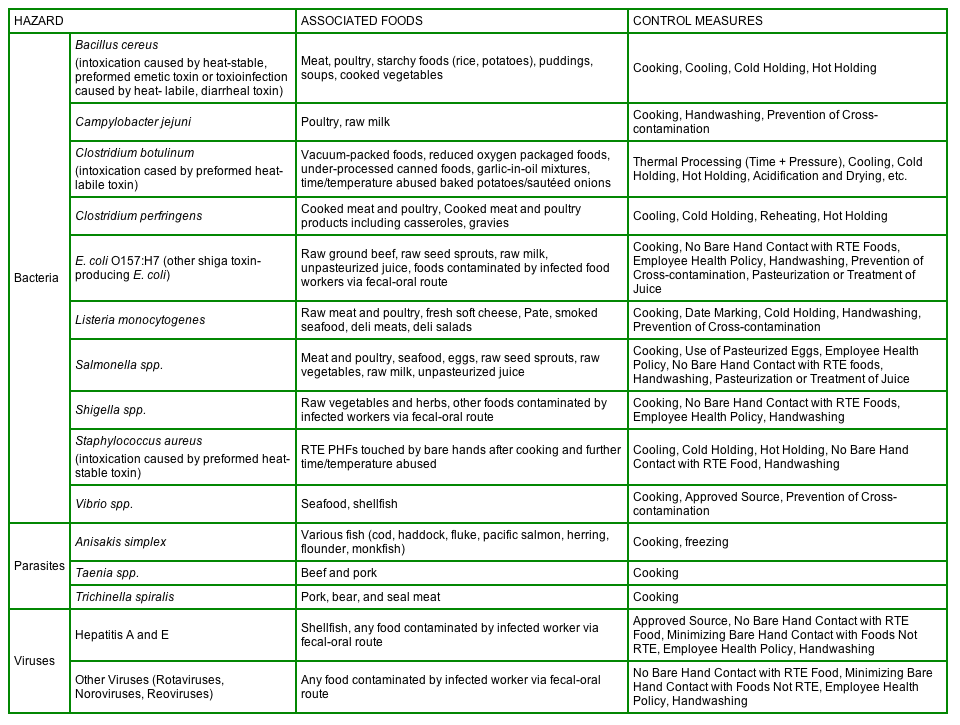
Below is a table taken from Annex 3 in Hazard Analysis, Managing Food Safety: A Regulator's Manual For Applying HACCP Principles to Risk-based Retail and Food Service Inspections and Evaluating Voluntary Food Safety Management Systems. Included are selected biological hazards found at retail, the associated foods, and control measures to address these hazards.
The Code provides specific measurable criteria referred to as critical limits, which are designed to prevent, eliminate, or reduce hazards in foods. The critical limits are based on the best available science and pertain to control measures applied within operational steps. They are established in the cooking, cooling, and reheating requirements and are mainly found in Chapter 3 of the Code.

Specific food processes that require a variance have historically resulted in more foodborne illness than standard processes. They present a significant health risk if not conducted under strict operational procedures. These types of operations may require the person in charge and food employees to use specialized equipment and demonstrate specific competencies. The variance requirement is designed to ensure that the proposed method of operation is carried out safely. The Code provides some flexibility with regulatory approval for food establishments in terms of adhering to Code regulations via variances and HACCP plans. In some cases the Code requires a preapproved variance and HACCP plan and in others only the availability of a HACCP plan and adherence to it (see §3-502.11 vs. §3-502.12).
The image below is taken from §8-201.13 of the Code and specifies conditions under which a HACCP Plan is required.

According to §8-201.14, a food establishment that is required to have a HACCP plan must include the following in their plan and specifications:
There are seven annexes located at the back of the document. While they were not adopted by 590 and are not part of the codified portion of the Code, they still remain a tremendous resource intended for regulator use but of interest to all.
The annexes are provided specifically to assist the regulatory authority apply the provisions uniformly and effectively. It is, therefore, important for users to preview the subject and essence of each of the annexes before using the document. Some of the annexes (e.g., References, Public Health Reasons) are structured to present the information by the specific Food Code item number to which they apply. Other annexes provide information and materials intended to be helpful to the user such as model forms that can be used, a delineation of the principles of HACCP, guidelines for establishment inspection, and criteria for certain food processes for use in evaluating proposed HACCP plans.
Annex 1 - Compliance and Enforcement
The purpose of this Annex is to set forth provisions, in codified form, that provide a full array of enforcement mechanisms while recognizing the diverse statutes and regulations that currently govern the operations of the thousands of state and local regulatory agencies.
Annex 2 - References
The Code makes frequent reference to federal statutes contained in the United States Code (USC) and the Code of Federal Regulations (CFR). Copies of the USC and CFR can be viewed and copied at government depository libraries or may be purchased as follows.
Annex 3 - Public Health Reasons / Administrative Guidelines
This annex can serve as a tremendous resource since it provides the public health rationale for the food safety provisions set forth in Chapters 2-7.
Annex 4 - Food Establishment Inspection
This annex provides regulatory agencies with guidance on planning, scheduling, conducting, and evaluating inspections. It also supports programs by providing recommendations for training and equipping the inspection staff, and attempts to enhance the effectiveness of inspections by stressing the importance of communication and information exchange during regulatory visits.
Annex 5 - HACCP Guidelines
This annex describes in details the seven principles of HACCP and how they can be utilized to develop an effective HACCP plan.
Annex 6 - Food Processing Criteria
The purpose of this Annex is to provide processing criteria for different types of food manufacturing/processing operations for use by those preparing and reviewing HACCP plans and proposals. Reduced Oxygen Packaging (ROP) and Smoking and Curing criteria are described in this annex.
Annex 7 - Model Forms, Guides, and Other Aids
The documents provided in this Annex are intended to facilitate adoption of the Code and the application of its provisions as they relate to applicants' and food employees' health and to food establishment inspections.
The Code contains a section known as the Summary of Changes that provides an outline of the textual changes from the 1997 FDA Food Code Chapters and Annexes to the 1999 edition. Every updated version of the Code contains a summary of changes from the previous edition. The Code revision process is an all inclusive process and involves all stakeholders, including federal, state and local regulators, industry, academia, and consumer representatives.
FDA is issuing a new edition of the Food Code every 4 years. During the 4-year span of time between editions, FDA may issue supplements to an existing edition. Each new edition will incorporate the changes made in the supplement as well as any new revisions. While FDA receives concerns and recommendations for modification of the Food Code from any individual or organization, there is a particular interest in addressing problems identified by those in government and industry who are responsible for implementing the Food Code. FDA is especially responsive to those needed policy and technical changes raised by an organization that uses a democratic process for addressing problems and concerns. This includes organizations that provide a process to encourage representative participation in deliberations by government, industry, and academic and consumer interests, followed by public health ratification such as a state-by-state vote by officially designated delegates. Examples of such organizations include:
These organizations receive problems submitted by any interested individual, but specify the forms on which the issues must be detailed and provide specific time frames during which they may be submitted. FDA encourages interested individuals to consider raising issues and suggesting solutions involving the federal-state cooperative programs based on FDA's model codes through these organizations.
While federal, state, and local governments are primarily responsible for setting food safety standards, there are many other organizations that share a stake in carrying out enforcement of the standards. The Conference for Food Protection (CFP) addresses this by bringing together representatives from the food industry, government, academia, and consumer organizations to identify and address emerging problems of food safety and to formulate recommendations. The CFP meets at least biennially to provide a forum for the consideration of ideas from any of the stakeholders. Although it has no formal regulatory authority, CFP is a powerful organization that greatly influences model laws and regulations among all government agencies.
The Code revision process begins when regulators, industry, or other stakeholders submit an issue that will be reviewed by the Councils. Separate committees in each discipline may be appointed to study and review issues and make recommendations to each Council at succeeding biennial meetings. The Councils then report out to the Voting Assembly where each state has one vote (which may be divided amongst several state agencies or voted by one agency). The CFP Recommendations are officially transmitted to FDA and the Agency responds as to areas of agreement or non-concurrence. Those positions may be further explored with the CFP Executive Board, but if FDA cannot agree with a Recommendation, it is not reflected in the Code. All accepted Recommendations are addressed in the next issue of the Code.
A few items in the Code are identified as "Reserved". This indicates an area of concern by FDA that was not supported by the CFP. However, it is maintained as reserved in the Code so that FDA can revisit the issue based on future information.
§5-203.15 was reserved in the 1999 Code.

As of the 2001 Code, §5-203.15 is no longer reserved.

FDA 1999 Food Code
Local Public Health Institute Orientation Modules: Food Protection
Managing Food Safety: A Manual for the Voluntary Use of HACCP Principles for Operators of Food Service and Retail Establishments
Managing Food Safety: A Regulator's Manual For Applying HACCP Principles to Risk-based Retail and Food Service Inspections and Evaluating Voluntary Food Safety Management Systems
State Sanitary Code Chapter X - Minimum Sanitation Standards for Food Establishments