Air Pollution
re of gases and a variety of naturally occurring and made-made particles and contaminants. The gases in air are:
- Nitrogen (76% by weight)
- Oxygen (23%)
- Argon (1%)
- Carbon dioxide (0.03%)
- and a variety of other gases in small quantity plus water vapor, depending on the humidity
Air pollutants are the particles, vapors, and contaminants not found in pure air. Pollutants can be naturally occurring or the result of human activity (anthropogenic).
|
Naturally Occurring Pollution |
Man-made Pollution |
|---|---|
|
|
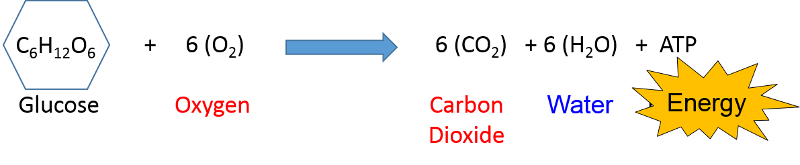
Air pollution is primarily the result of burning hydrocarbon fuels and releasing the by-products into the atmosphere. At the beginning of this module it was noted that cellular metabolism involves the oxidation of glucose or fat (hydrocarbon compounds) in order to release energy in the form of ATP that can be used for a variety of cellular processes that require energy.
involves the oxidation of glucose or fat (hydrocarbon compounds) in order to release energy in the form of ATP that can be used for a variety of cellular processes that require energy.
Pollution from Combustion of Fuels
The energy that humans require for heat, electricity, automobiles, industry etc. is mostly derived by combustion of hydrocarbon fuels. Combustion also involves oxygen combining with hydrocarbonsl (gasoline, oil, coal, propane, natural gas, wood, diesel fuel, etc.) to produce energy. The by-products are carbon dioxide (CO2), carbon monoxide (CO), water vapor, smoke, and ash (particulate residue). Carbon monoxide (CO) tends to be formed when there is insufficient oxygen for the hydrocarbon to be oxidized (burned) completely. Nitrogen is the most abundant gas in the air we breath, and it too combines with oxygen during combustion to form a series of compounds called nitrogen oxides (NO2, N20, NO); these are sometimes collectively referred to as "NOx." Sulfur dioxide (SO2) is also formed during combustion of sulfur-containing fuels such as coal or unrefined oil. Combustion of hydrocarbon fuels also produces "particulates", some of which are visible as smoke and some are so small that they are invisible. As we will see, these by-products of combustion pose threats to health and to the environment.
of hydrocarbon fuels. Combustion also involves oxygen combining with hydrocarbonsl (gasoline, oil, coal, propane, natural gas, wood, diesel fuel, etc.) to produce energy. The by-products are carbon dioxide (CO2), carbon monoxide (CO), water vapor, smoke, and ash (particulate residue). Carbon monoxide (CO) tends to be formed when there is insufficient oxygen for the hydrocarbon to be oxidized (burned) completely. Nitrogen is the most abundant gas in the air we breath, and it too combines with oxygen during combustion to form a series of compounds called nitrogen oxides (NO2, N20, NO); these are sometimes collectively referred to as "NOx." Sulfur dioxide (SO2) is also formed during combustion of sulfur-containing fuels such as coal or unrefined oil. Combustion of hydrocarbon fuels also produces "particulates", some of which are visible as smoke and some are so small that they are invisible. As we will see, these by-products of combustion pose threats to health and to the environment.
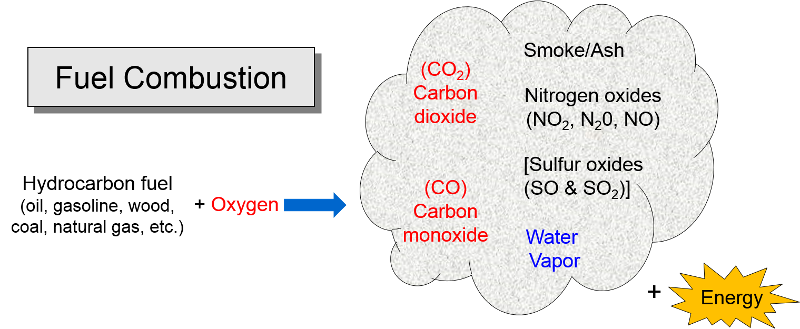
Other Pollutants from Combustion
Other pollutants can also be released by combustion, depending on the combustion of what is being burned. Sulfur dioxide is present in coal and crude oil, but sulfur is removed when oil is refined to produce gasoline, so coal is the primary source of SO2.

Source: National Geographic
http://environment.nationalgeographic.com/environment/global-warming/pollution-overview/
Mercury
Coal also contains small concentrations of elemental mercury which vaporizes when coal is burned. The vapors can remain in the atmosphere for some time and travel with wind currents until the mercury eventually returns to earth during precipitation. Some bacteria are able to convert elemental mercury into methylmercury, an organic form which can be taken up by algae. Methylmercury then moves up through the food chain from algae to zooplankton and then to fish of increasing size. Through this process of bioaccumulation and biomagnification mercury can be found in the high concentrations large saltwater such as tuna and swordfish. Fetuses, infants and small children exposed to methylmercury can have severely impaired neurological development. For more information see the EPA web page on health effects of mercury.
and then to fish of increasing size. Through this process of bioaccumulation and biomagnification mercury can be found in the high concentrations large saltwater such as tuna and swordfish. Fetuses, infants and small children exposed to methylmercury can have severely impaired neurological development. For more information see the EPA web page on health effects of mercury.
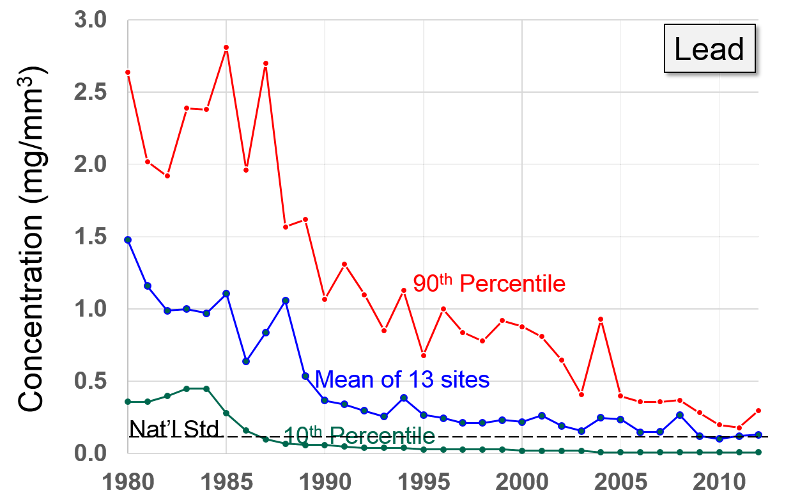
Lead
Lead is another toxic heavy metal that enter the atmosphere during combustion of materials in which it is present. In the 1920s gasoline producers began adding lead in order to reduce "engine knock" . The combustion of gasoline in vehicles using leaded gas produced high levels of lead in the atmosphere. As the health hazards of lead became increasingly apparent, the use of lead additives declined, and they were banned in the US in 1995. Most other countries have subsequently banned lead additives. At present industrial sources (e.g. smelters, battery manufacture, metal processing) are responsible for the majority of lead in the atmosphere. Lead is also neurotoxic and can cause a spectrum of problems ranging from subtle impairments in development to severe impairments in nerve function, learning, and hearing. It can also cause anemia and, in severe cases death. For more information see the EPA web page on health effects of lead.
. The combustion of gasoline in vehicles using leaded gas produced high levels of lead in the atmosphere. As the health hazards of lead became increasingly apparent, the use of lead additives declined, and they were banned in the US in 1995. Most other countries have subsequently banned lead additives. At present industrial sources (e.g. smelters, battery manufacture, metal processing) are responsible for the majority of lead in the atmosphere. Lead is also neurotoxic and can cause a spectrum of problems ranging from subtle impairments in development to severe impairments in nerve function, learning, and hearing. It can also cause anemia and, in severe cases death. For more information see the EPA web page on health effects of lead.
Volatile Organic Compounds (VOCs)
VOCs are organic compounds (i.e., hydrocarbons) that evaporate readily at room temperature and enter the atmosphere. VOCs are in present gasoline and other fuels and in many industrial and household products, such as organic solvents, cleaning supplies, paint, lacquer, varnish, paint strippers, cleaning supplies, pesticides, building materials, copiers and printers. VOCs are important participants in the generation of ozone, which is a secondary pollutant which is discussed below in the section on Criteria Pollutants.
Polycyclic Aromatic Hydrocarbons (PAHs)
PAHs represent a large family of molecules that can be naturally occurring, but they are also produced when coal, oil, gas, and garbage are burned incompletely. PAHs can also be generated by cooking meats at high temperatures. One can be exposed to PAHs in the air, or they can be ingested in contaminated food or water, and also by direct contact with PAH-contaminated soil or products like heavy oils, coal tar, roofing tar, or creosote. PAHs cause tumors and birth defects in rodents exposed to PAHs through food inspired inspired air, or direct contact with skin.
Examples of PAHs
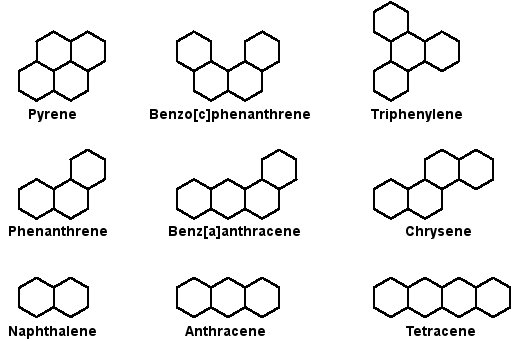
Image source: http://www.astrochem.org/sci/Cosmic_Complexity_PAHs.php
In the video clip below (<3 min.), Dr. David Sherr, from the environmental health department at BUSPH, discusses the potential role of environmental pollutants as risk factors for breast (and other) cancers. Dr. Sherr's research is exploring the molecular mechanisms that initiate and maintain breast cancer. Much of his research has focused on the role of an environmental chemical receptor (the aryl hydrocarbon receptor - AhR) and the role of polycyclic aromatic hydrocarbons (PAHs), which are atmospheric pollutants generated by burning fuel and by cooking meat at high temperatures (e.g., grilling). PAHs are also found in smoked fish. In this video, Dr. Sherr also discusses the difficulties in establishing a causal link between environmental pollutants and cancer.
The Six Criteria Pollutants
From the EPA (http://www.epa.gov/airquality/urbanair/):
"The Clean Air Act requires EPA to set National Ambient Air Quality Standards for six common air pollutants. These commonly found air pollutants (also known as "criteria pollutants") are found all over the United States. They are particle pollution (often referred to as particulate matter), ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. These pollutants can harm your health and the environment, and cause property damage. Of the six pollutants, particle pollution and ground-level ozone are the most widespread health threats. EPA calls these pollutants "criteria" air pollutants because it regulates them by developing human health-based and/or environmentally-based criteria (science-based guidelines) for setting permissible levels. The set of limits based on human health is called primary standards. Another set of limits intended to prevent environmental and property damage is called secondary standards.
Click on one of the pollutants below for information on sources of the pollutant, why the pollutant is of concern, health and environmental effects, efforts underway to help reduce the pollutant, and other helpful resources."
|
Sources of Air Pollution Data from http://www.epa.gov/air/emissions/index.htm |
Health Effects of Criteria Pollutants From http://www.epa.gov/region7/air/quality/health.htm |
Pollutant Concentrations Over Time Data from http://www.epa.gov/airtrends/index.html |
|
|
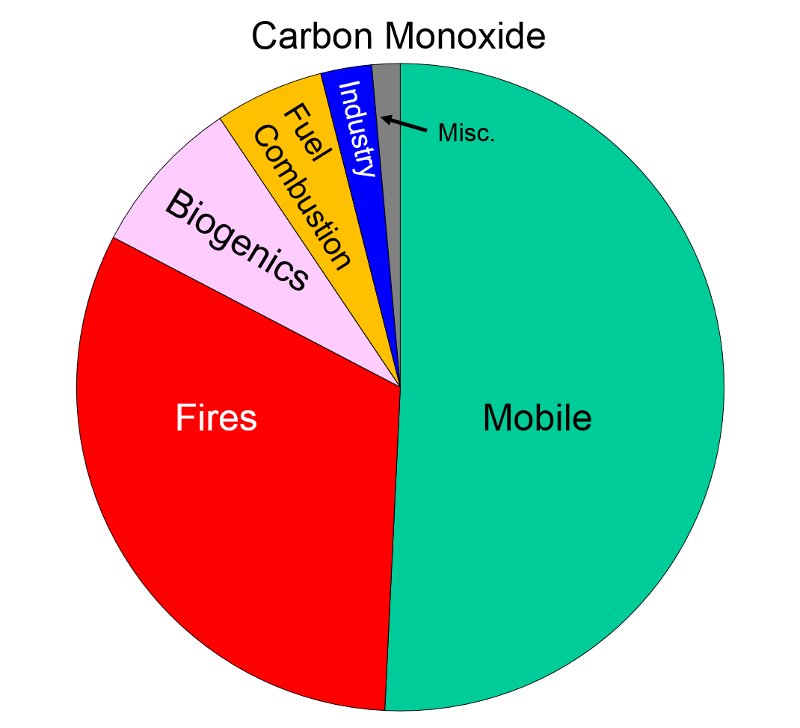
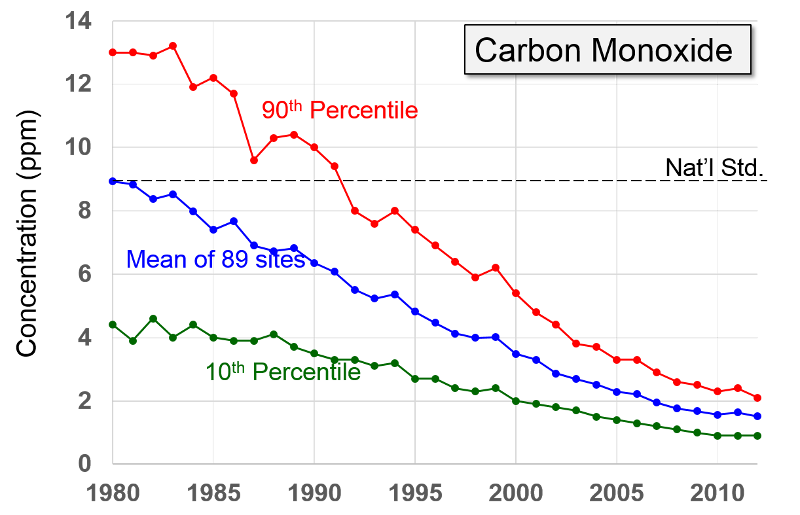
Carbon Dioxide "Carbon monoxide is a colorless odorless poisonous gas formed when carbon in fuels is not burned completely. It is a byproduct of motor vehicle exhaust, which contributes more than two-thirds of all CO emissions nationwide. In cities, automobile exhaust can cause as much as 95 percent of all CO emissions."
"Carbon monoxide enters the bloodstream and reduces oxygen delivery to the body's organs and tissues. The health threat from CO is most serious for those who suffer from cardiovascular disease. Healthy individuals are also affected, but only at higher levels of exposure. Exposure to elevated CO levels is associated with visual impairment, reduced work capacity, reduced manual dexterity, poor learning ability, and difficulty in performing complex tasks."
NOTE: High concentrations, e.g., from a car running in an unvented garage or from poorly vented kerosene heaters, can be fatal.
See also CO in Smokers |
|
|
|
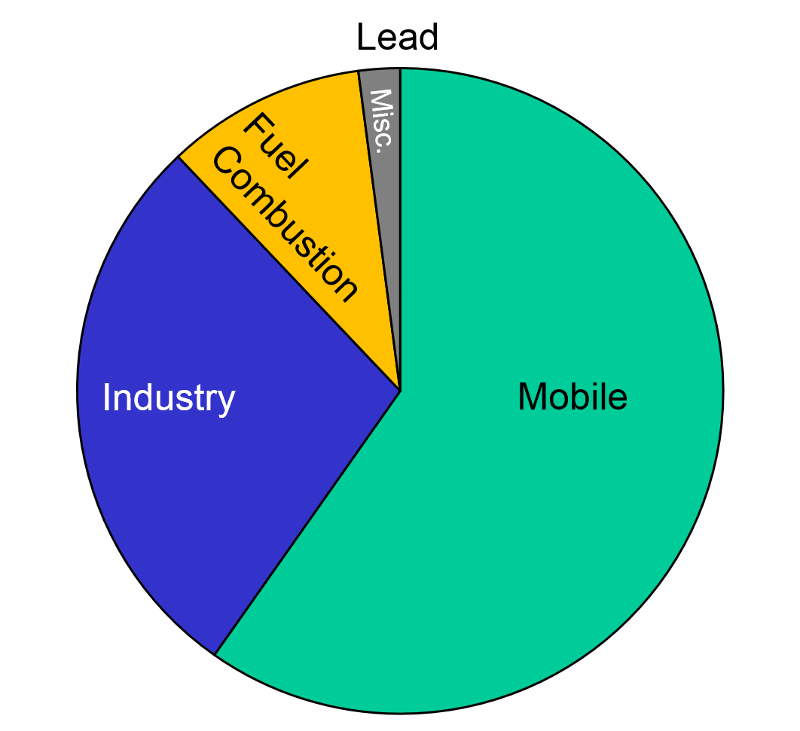
Lead "Exposure to lead mainly occurs through inhalation of air and ingestion of lead in food, paint, water, soil, or dust. Lead accumulates in the body in blood, bone, and soft tissue. Because it is not readily excreted, lead can also affect the kidneys, liver, nervous system, and other organs. Excessive exposure to lead may cause anemia, kidney disease, reproductive disorders, and neurological impairments such as seizures, mental retardation, and/or behavioral disorders. Even at low doses, lead exposure is associated with changes in fundamental enzymatic, energy transfer, and other processes in the body. Fetuses and children are especially susceptible to low doses of lead, often suffering central nervous system damage or slowed growth. Recent studies show that lead may be a factor in high blood pressure and subsequent heart disease in middle-aged white males. Lead may also contribute to osteoporosis in postmenopausal women."
After banning lead additives in gasoline, the primary source of lead in the has become smelters and battery plants. The highest concentrations of lead are found in the vicinity of nonferrous smelters and other stationary sources of lead emissions.
|
|
|
|
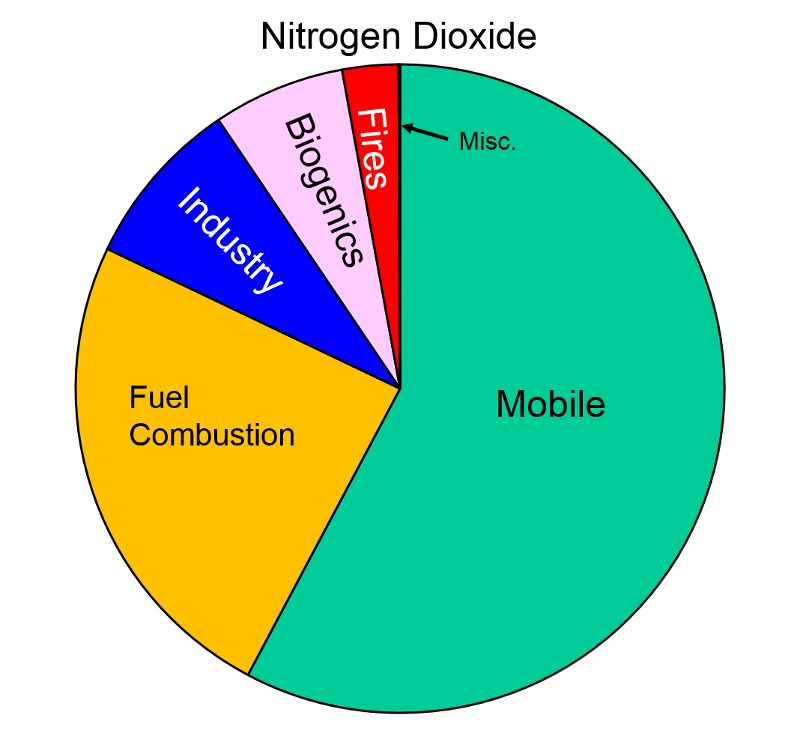
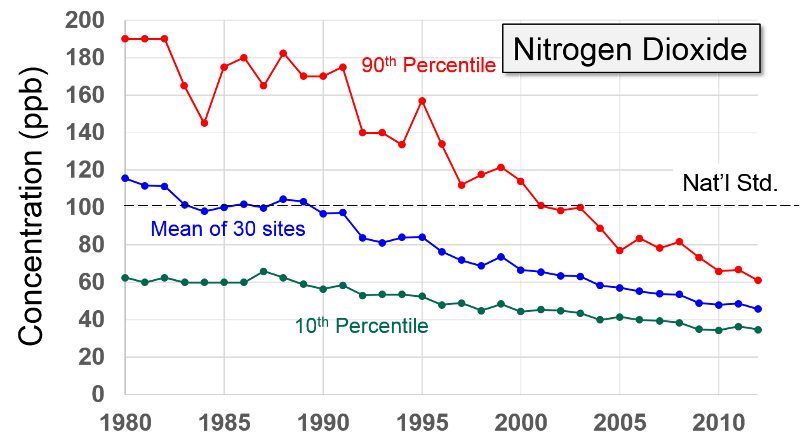
Nitrogen Oxides Nitrogen oxides form when fuel is burned at high temperatures and come principally from motor vehicle exhaust and stationary sources such as electric utilities and industrial boilers. A suffocating, brownish gas, nitrogen dioxide is a strong oxidizing agent that reacts in the air to form corrosive nitric acid, as well as toxic organic nitrates. It also plays a major role in the atmospheric reactions that produce ground-level ozone (or smog).
Health and Other Effects: Nitrogen dioxide can irritate the lungs and lower resistance to respiratory infections such as influenza. The effects of short-term exposure are still unclear, but continued or frequent exposure to concentrations that are typically much higher than those normally found in the ambient air may cause increased incidence of acute respiratory illness in children. EPA's health-based national air quality standard for NO2 is 0.053 ppm (measured as an annual average). Nitrogen oxides are important in forming ozone and may affect both terrestrial and aquatic ecosystems. Nitrogen oxides in the air are a potentially significant contributor to a number of environmental effects such as acid rain and eutrophication in coastal waters like the Chesapeake Bay. Eutrophication occurs when a body of water suffers an increase in nutrients that reduce the amount of oxygen in the water, producing an environment that is destructive to fish and other animal life."
|
|
|
|
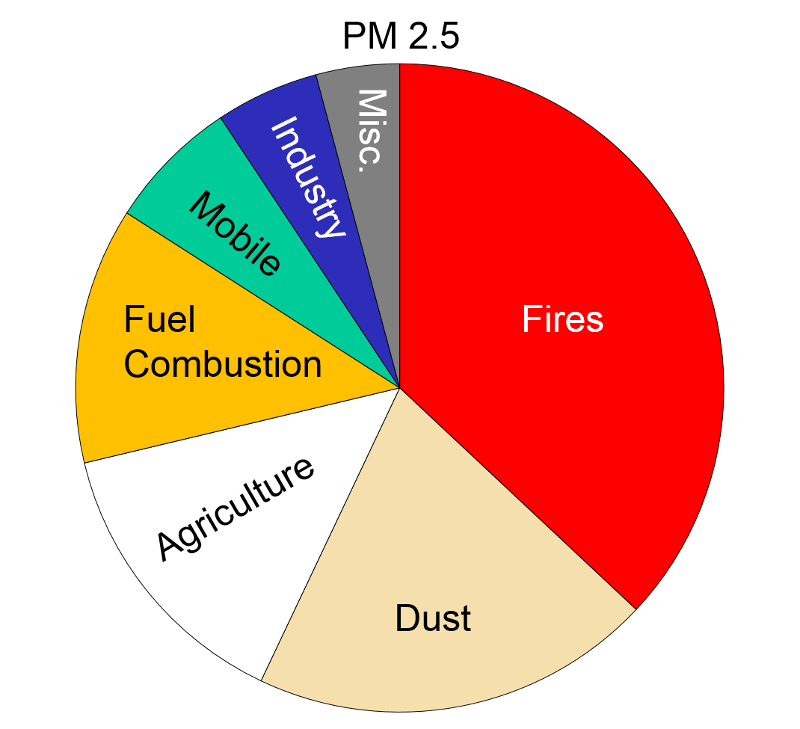
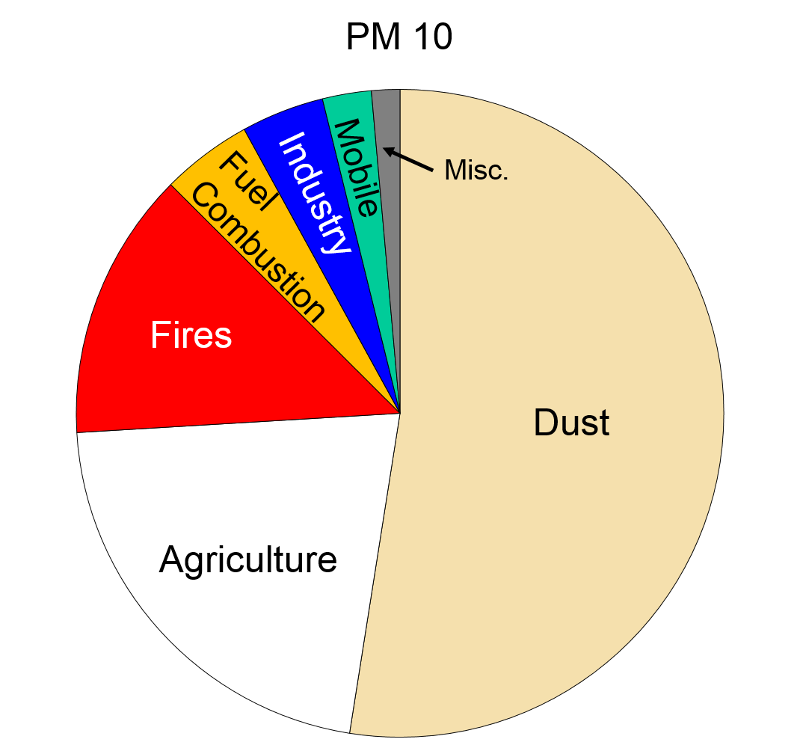
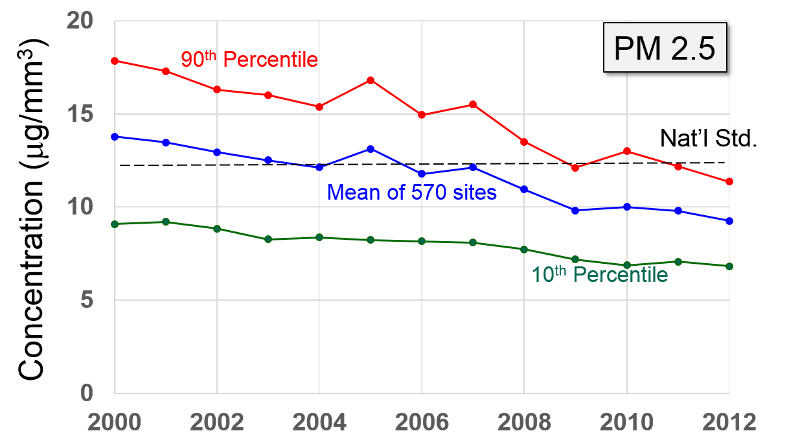
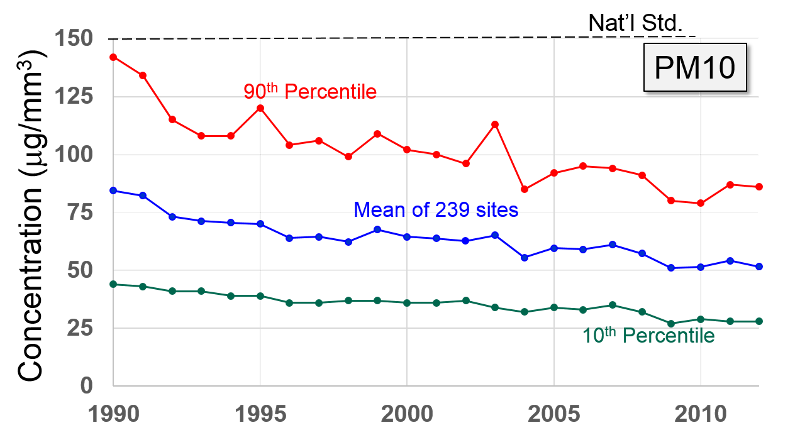
Particulate Matter (PM2.5 and PM10) "Particulate matter is the term for solid or liquid particles found in the air. Some particles are large or dark enough to be seen as soot or smoke. Others are so small they can be detected only with an electron microscope. Because particles originate from a variety of mobile and stationary sources (diesel trucks, wood stoves, power plants, etc.), their chemical and physical compositions vary widely."
Particles less than 10 microns (micrometers) in diameter can be inhaled, and these are divided into PM-10 and PM2.5. PM-10 includes particles with a diameter of 10 microns or less; these tend to be generated by wind or mechanical agitation from plowing, grinding, etc. Particles between 2.5-10 microns tend to adhere to the mucus in the trachea and bronchi; they are then gradually swept up to the pharynx by the cilia on epithelial cells lining the respiratory tract. Once they reach the pharynx, they can be swallowed or expectorated.
PM-2.5 particles with a diameter of 2.5 microns or less, often referred to as "fine particulates", are mostly generated from combustion. Ultrafine particles, <0.1 microns, are generated from diesel engines, particularly when they are idling. Particles <2.5 microns are able to reach the lower respiratory tract and alveoli because they are small and light and can be carried easily with the inspired air.
Health Effects: "Major concerns for human health from exposure to particulate matter are: effects on breathing and respiratory systems, damage to lung tissue, cancer, and premature death. The elderly, children, and people with chronic lung disease, influenza, or asthma, tend to be especially sensitive to the effects of particulate matter. Acidic particulate matter can also damage manmade materials and is a major cause of reduced visibility in many parts of the U.S. Click here for more information on the health effects of particulate matter."
|
|
|
|
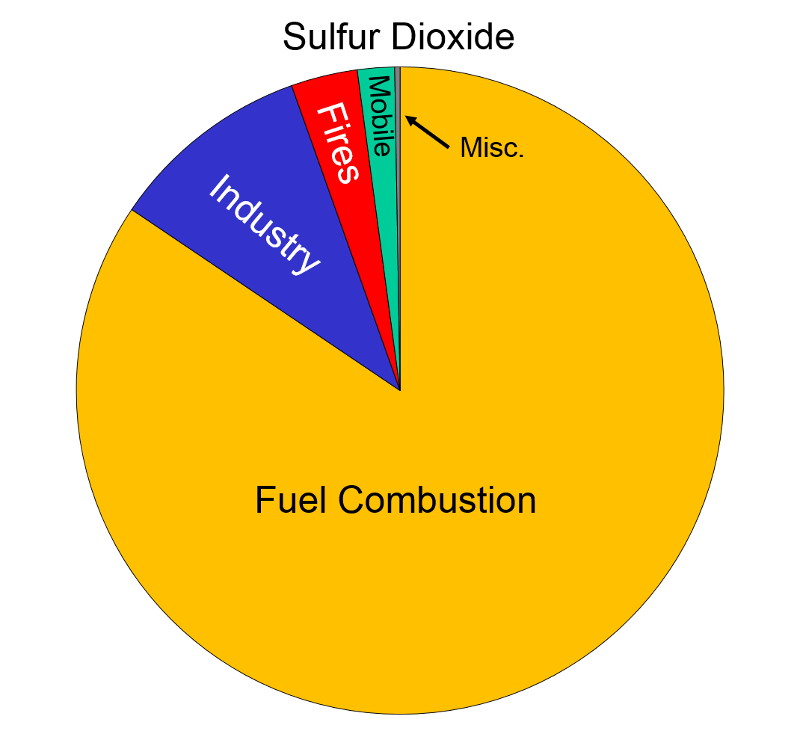
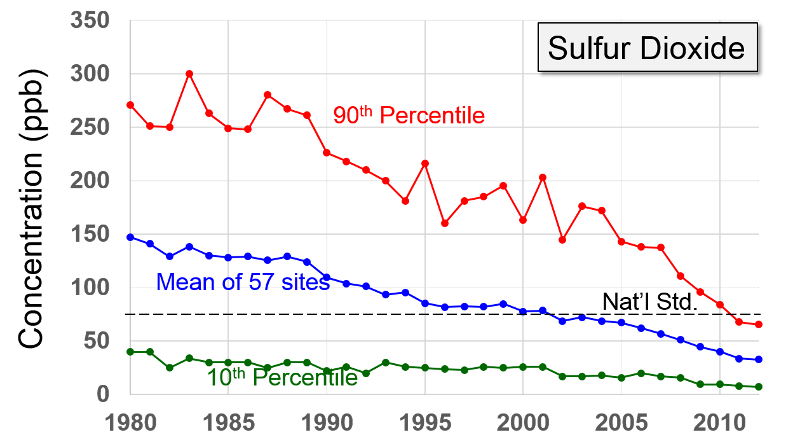
Sulfur Oxides "These gases are formed when fuel containing sulfur (mainly coal and oil) is burned, and during metal smelting and other industrial processes.
Health and Other Effects: The major health concerns associated with exposure to high concentrations of SO2 include effects on breathing, respiratory illness, alterations in pulmonary defenses, and aggravation of existing cardiovascular disease. Major subgroups of the population that are most sensitive to SO2 include asthmatics and individuals with cardiovascular disease or chronic lung disease (such as bronchitis or emphysema) as well as children and the elderly. EPA's health-based national air quality standard for SO2 is 0.03 ppm (measured on an annual average) and 0.14 ppm (measured over 24 hours). Emissions of SO2 also can damage the foliage of trees and agricultural crops. EPA has a secondary SO2 national ambient air quality standard of 0.50 ppm (measured over 3 hours) designed to prevent this type of environmental deterioration. Together, SO2 and NOX are the major precursors to acid rain, which is associated with the acidification of lakes and streams, accelerated corrosion of buildings and monuments, and reduced visibility."
|
|
|
|
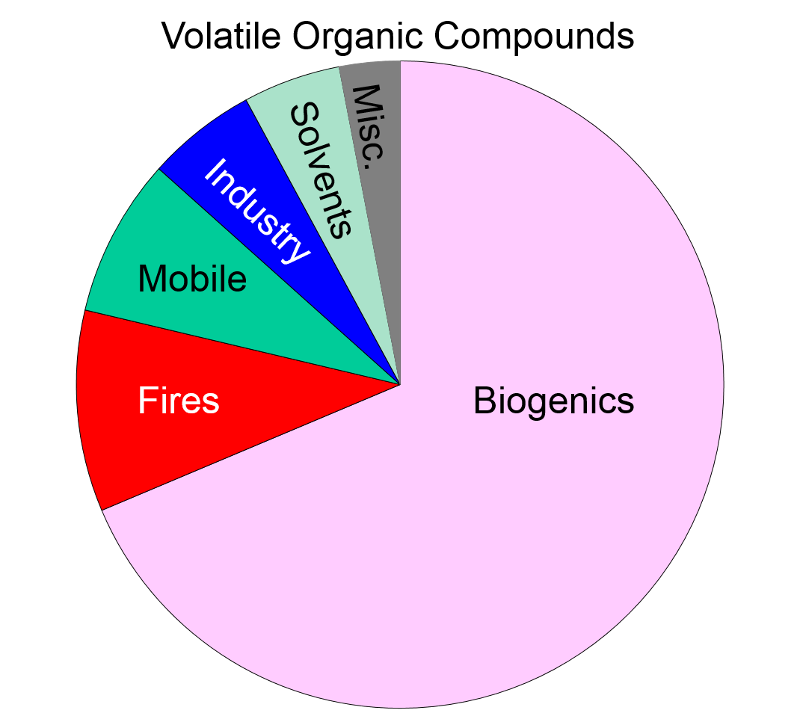
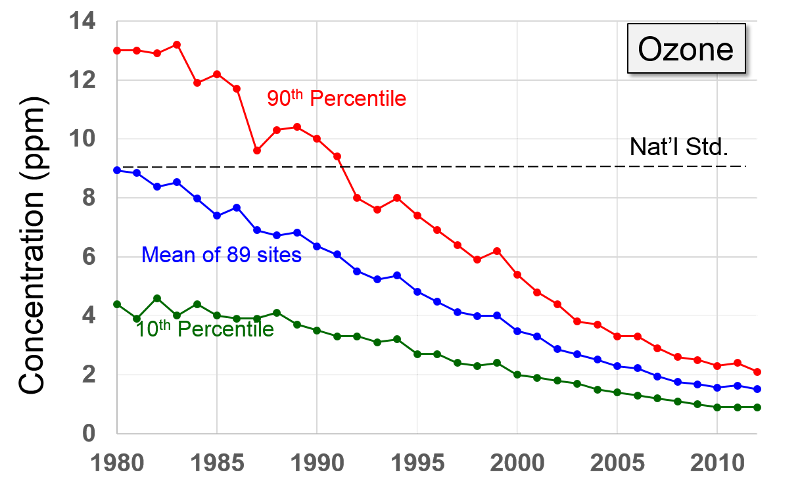
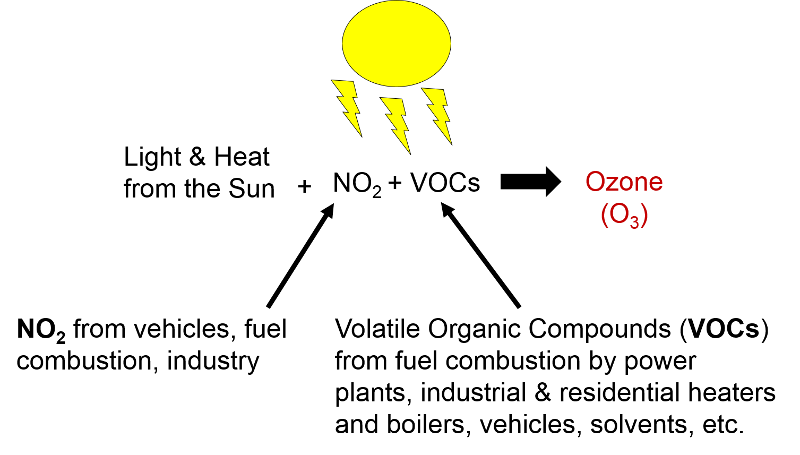
Volatile Organic Compounds "Unlike other pollutants, ozone is not emitted directly into the air by specific sources. Ozone is created by sunlight acting on nitrogen oxides (NOx) and volatile organic compound (VOC) emissions in the air. There are literally thousands of sources of these gases. Some of the more common sources include gasoline vapors, chemical solvents, combustion products of various fuels, and consumer products. They can originate from large industrial facilities, gas stations, and small businesses such as bakeries and dry cleaners. Often these "precursor" gases are emitted in one area, but the actual chemical reactions, stimulated by sunlight and temperature, take place in another. Combined emissions from motor vehicles and stationary sources can be carried hundreds of miles from their origins, forming high ozone concentrations over very large regions. Health and Other Effects: Scientific evidence indicates that ground-level ozone not only affects people with impaired respiratory systems (such as asthmatics), but healthy adults and children as well. Exposure to ozone for 6 to 7 hours, even at relatively low concentrations, significantly reduces lung function and induces respiratory inflammation in normal, healthy people during periods of moderate exercise. It can be accompanied by symptoms such as chest pain, coughing, nausea, and pulmonary congestion. Recent studies provide evidence of an association between elevated ozone levels and increases in hospital admissions for respiratory problems in several U.S. cities. Results from animal studies indicate that repeated exposure to high levels of ozone for several months or more can produce permanent structural damage in the lungs. |
|
|
Ozone Formation The naturally occurring ozone in the stratosphere protects the earth from the damaging effects of ultraviolet radiation from the sun. However, so called ground level ozone that forms as a result of man-made pollution, is harmful to health. Ozone is produced in the atmosphere by a chemical reaction between nitrogen oxides, volatile organic compounds, and light and heat energy from the sun.
|
||
Summary of Health Effects of Air Pollution
- PM-10 are deposited in mucus layer of the trachea and bronchi; their primary effect is to damage the ciliated cells of the mucociliary escalator.
- PM-2.5 pose more of a health hazard since they can reach the lower respiratory tract and alveoli, where they can cause local inflammation and interfere with the function of alveolar macrophages, which are an important component of lung defenses against infection. Ultrafine particles can pass through the alveolar wall into the circulation.
- Ozone, SO2, and NO2 are gases that can be carried all the way to the alveoli. All three of these are highly reactive compounds that cause irritation and inflammation of the respiratory airways and the alveoli.
- Carbon monoxide reaches the alveoli where it crosses the alveolar wall into the bloodstream and binds to hemoglobin.
Temperature Inversions
The effects of air pollution are greatly magnified when temperature inversions, or weather inversions, occur. The video below on the right explains temperature inversions. Ordinarily, air temperature is greatest at the earth's surface and decrease as altitude increases. Certain weather patterns can result in a blanket of warm air being inserted above cooler air at the earth's surface (an inversion), and this can prevent air mixing and trap pollutants at ground level.
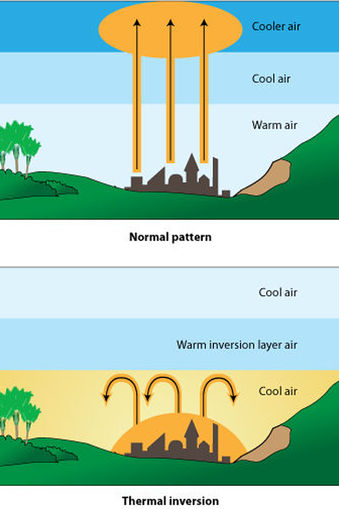
Image source http://www.sciencelearn.org.nz/Contexts/Enviro-imprints/Sci-Media/Images/Temperature-inversion
This video (3 min.) from the National Weather Service in Medford, Oregon, provides a succinct explanation of temperature inversions.
Source http://www.youtube.com/watch?v=L7i7N-je-aM
Air Quality Widget
The widget below shows the air quality for the 02118 zip code in Boston. To insert the widget into a web page for another zip code, paste the following HTML code into your web page:
<iframe height="200" width="310" src="http://epa.gov/cgi-bin/widget.cgi?ZIPCODE"></iframe>,
and insert the zip code of interest in place of ZIPCODE.