Innate Immunity
The Inflammatory Response
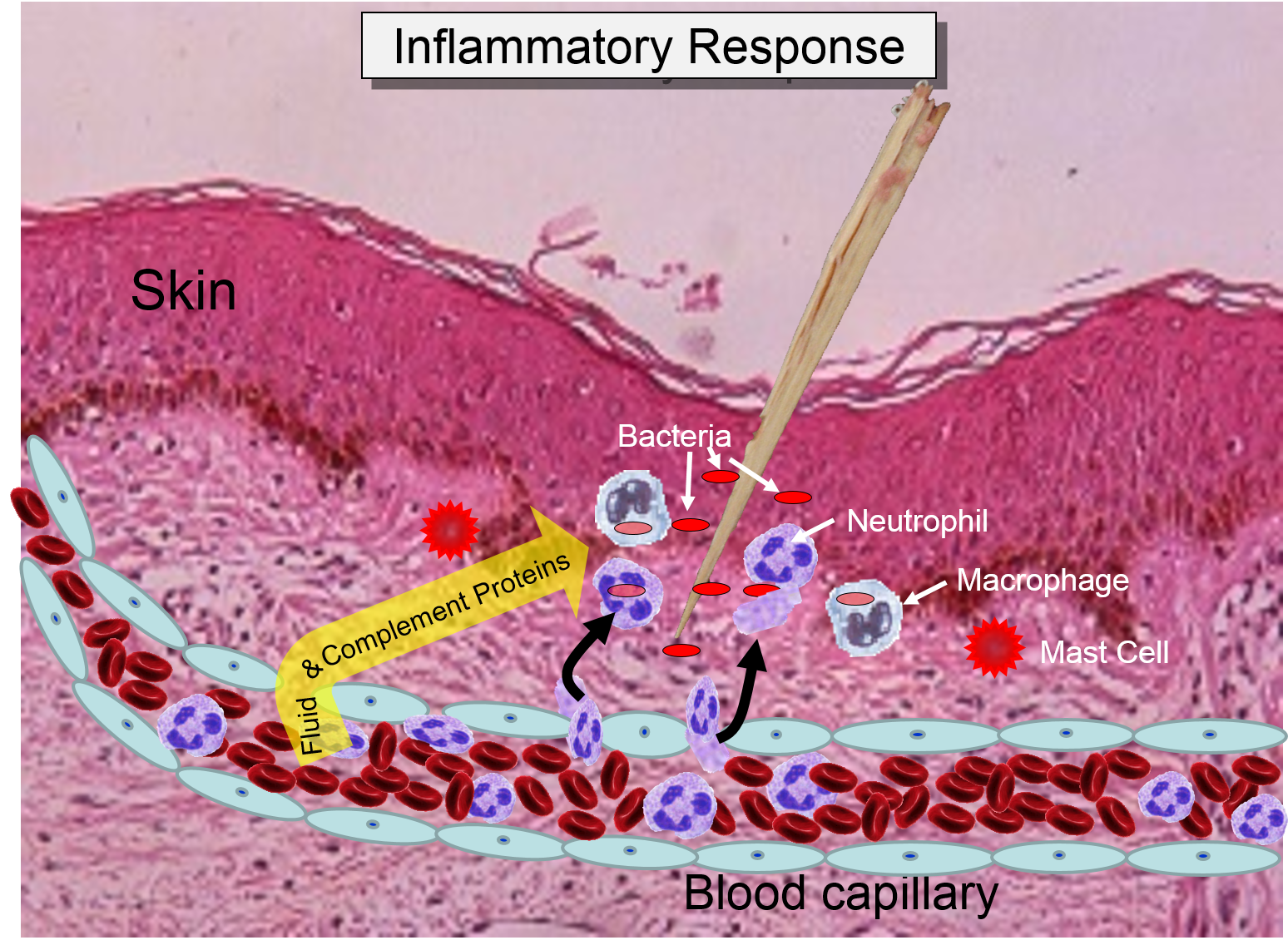
Injury or infection will an inflammatory response, which is an array of cellular events that is a major component of the innate immune response. To illustrate, consider the events that would be expected to take place after a splinter of wood sticks into your finger or you cut your foot on a piece of glass while walking barefooted. In both cases, the barrier function of the skin is breached, tissues are injured, and bacteria are introduced into the underlying tissues.
1) Binding of PAMPs to Toll-like Receptors
Injury to cells will prompt them to release chemical messengers, such as prostaglandins. In addition, the exterior surfaces of pathogens often have PAMPs on glycoproteins projecting from theirl surface or the flagella of motile pathogens. These PAMPs bind to toll-like receptors on macrophages, neutrophils, and dendritic cells, and they are also present on epithelial cells in the respiratory and gastrointestinal tracts. Binding of PAMPs to toll-like receptors in tissues is the alarm signal that triggers an inflammatory response, and PAMPs can also activate the complement system (which will be described later in this module.) Neutrophils are not normally present in tissues, unless there is injury or infection, but neutrophils constantly circulate in blood in large numbers. Nevertheless, macrophages are present in tissues, and always ready to respond.
2) Phagocytosis of Pathogens by Macrophages
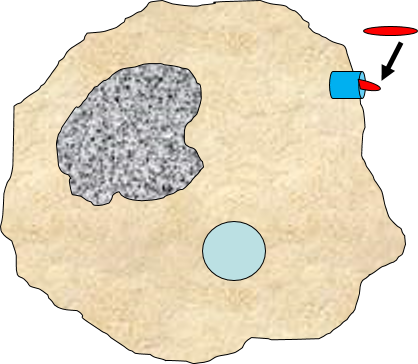
Binding of a pathogen's PAMPs to the toll-like receptors on tissue macrophages activates them and initiates phagocytosis of the pathogen. The sequence of drawings below illustrate the steps in phagocytosis as a bacterium binds to toll-like receptors and is then engulfed and digested.
of the pathogen. The sequence of drawings below illustrate the steps in phagocytosis as a bacterium binds to toll-like receptors and is then engulfed and digested.
|
A bacterium (red) binds to a toll-like receptor on a macrophage. |
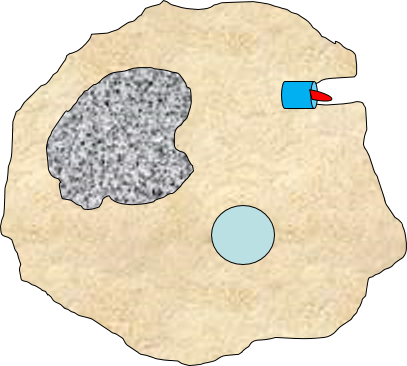
The cell begins to flow around the bacterium, gradually engulfing it. |
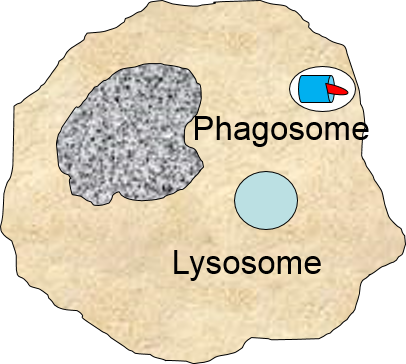
A portion of the cell membrane pinches off forming a phagosome. |
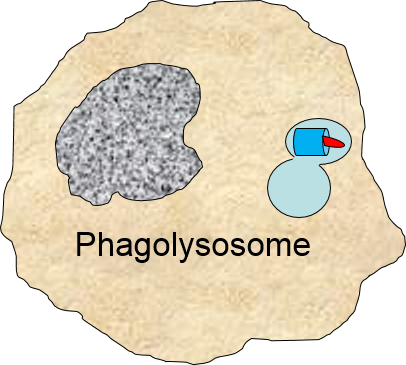
The phagosome fuses with a lysosome which contains digestive enzymes. |
The enzymes break the pathogen & receptor into pieces, & some antigen fragments are bound to antigen-presenting MHC II molecules. |
The residue of the bacterium is expelled by exocytosis, & the antigen-bearing MHC II molecule is inserted into the cell membrane. |
Note also that some of the molecular fragments of the bacterium are combined with newly-synthesized MHC Class II molecules in the endoplasmic reticulum. This complex is then inserted in the macrophage's cell membrane, effectively "displaying" the pathogenic antigens on its surface. This is an important mechanism by which macrophages alert the adaptive immune system to the presence of a foreign agent. This will be described in the section on adaptive immunity.
in the endoplasmic reticulum. This complex is then inserted in the macrophage's cell membrane, effectively "displaying" the pathogenic antigens on its surface. This is an important mechanism by which macrophages alert the adaptive immune system to the presence of a foreign agent. This will be described in the section on adaptive immunity.
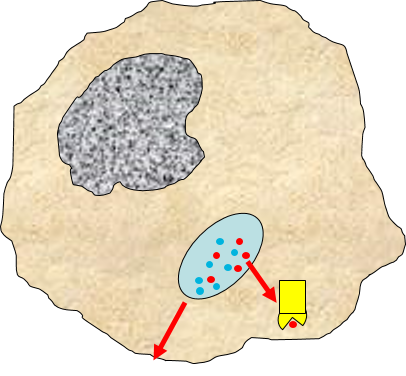
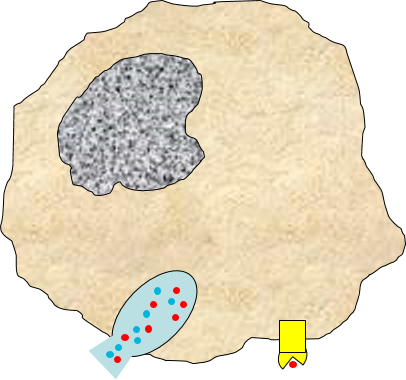
3) Signaling to Other Cells
Biochemical signals are released from injured cells (e.g., prostaglandins) and mast cells (e.g., histamine) and from macrophages. Activation of macrophages by binding of PAMPs stimulates the
synthesis and release of a variety of cytokines and chemokines
and chemokines .
.
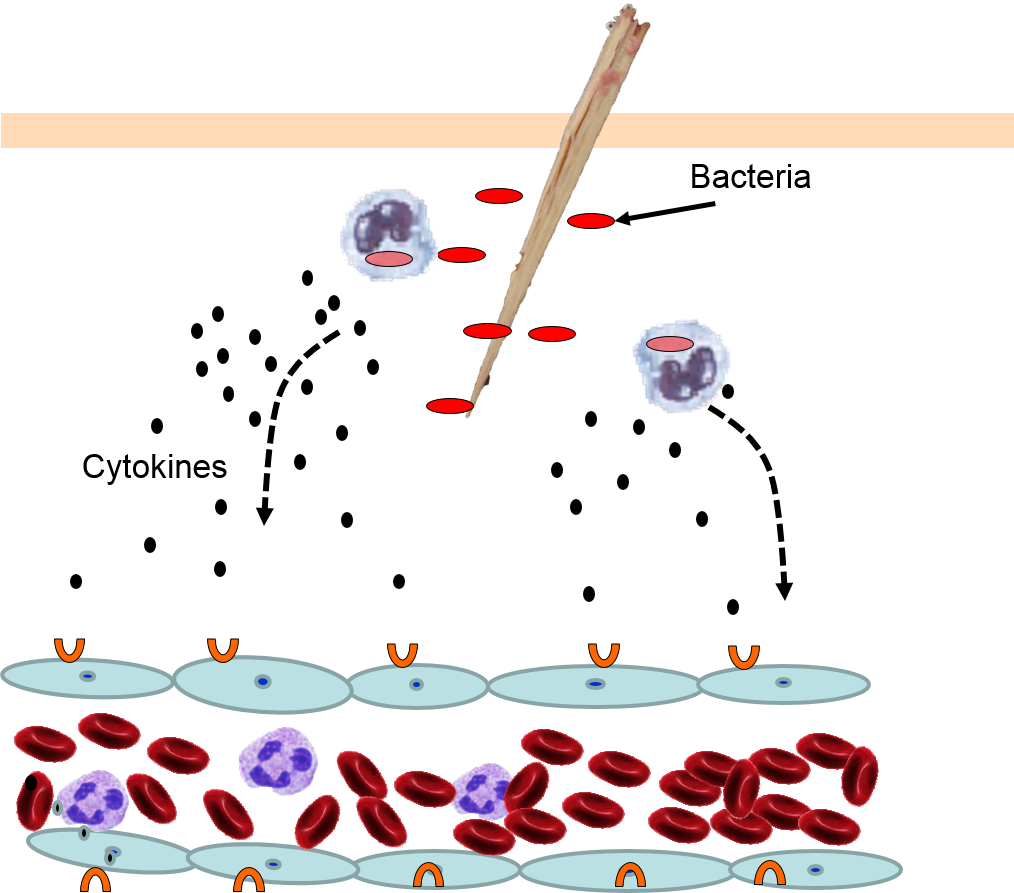
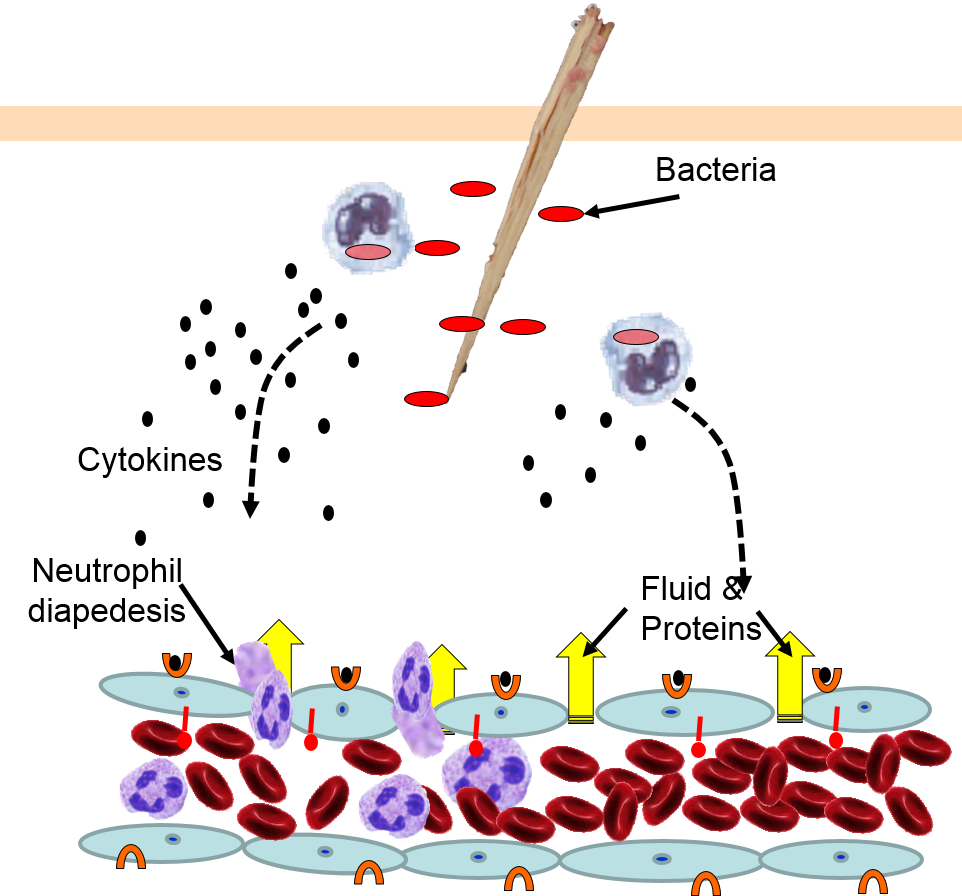
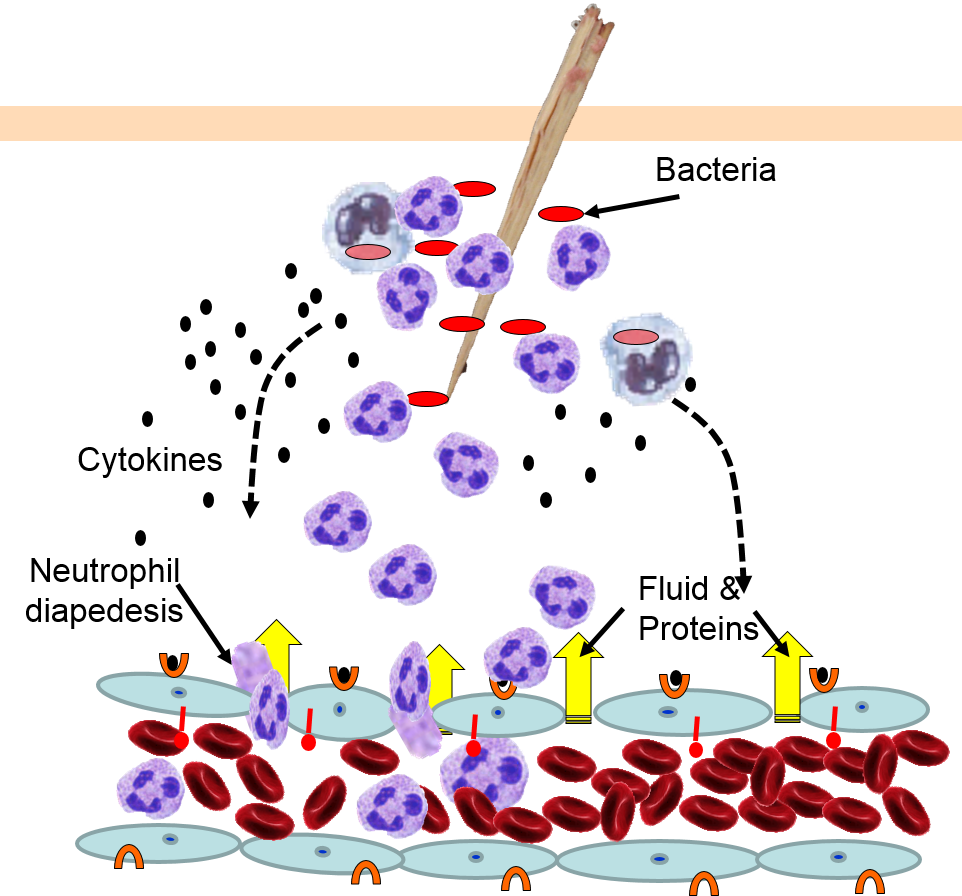
4) Changes in Local Blood Vessels
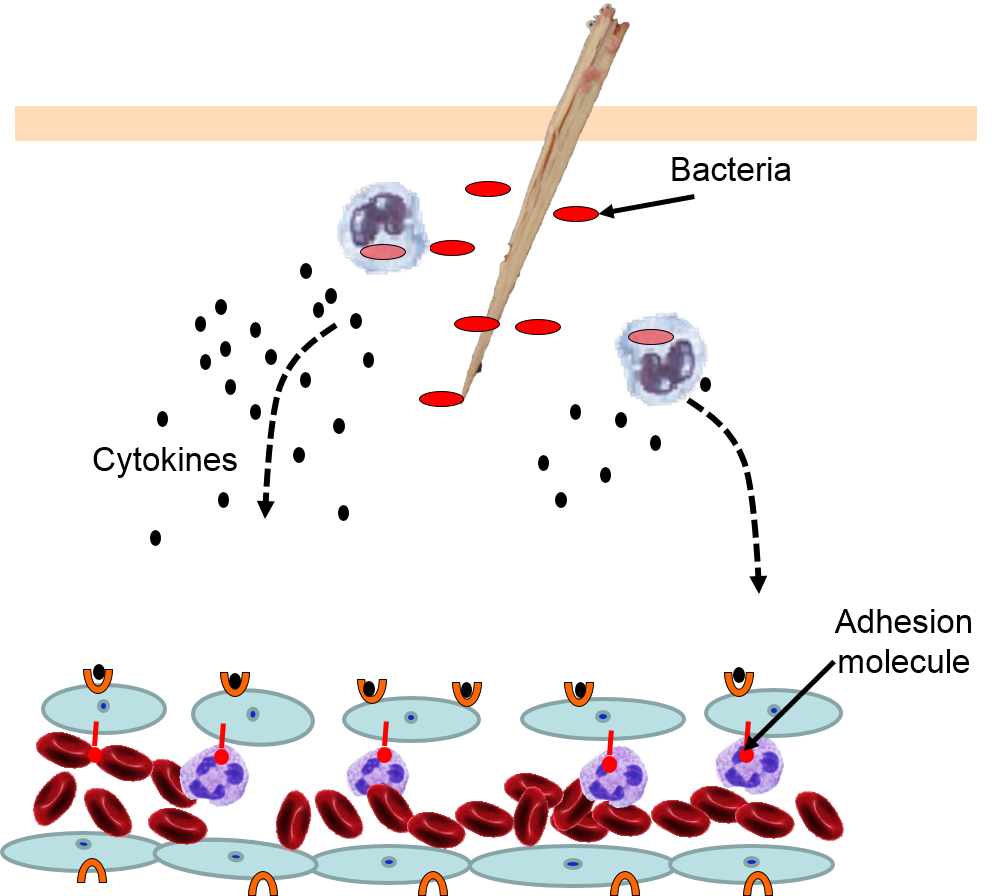
The various signaling molecules that are released after injury or infection (prostaglandins, histamine, and cytokines) induce important changes in local capillaries. Cytokines diffuse to local capillaries and bind to receptors which induce changes in the endothelial cells lining local capillaries, as depicted below.
|
Cytokines bind to receptors (orange semi-circles) on endothelial cells of a nearby capillary. This causes expression of adhesion molecules on the luminal surface of the endothelial cells (red lollipops), and causes endothelial cells to change shape, creating gaps between them. |
Neutrophils circulating in blood briefly bind to the adhesion molecules and then migrate between the endothelial cells (diapedesis) and following the trail of chemokines (not shown) toward the site of infection. The gaps in the endothelium also allow fluid and proteins from blood to enter the tissue. |
Neutrophils kill the invading bacteria by phagocytosis aided by complement proteins which tag the bacteria to facilitate identification and phagocytosis of the pathogens. After phagocytosing bacteria, the neutrophils die. If the number of dead neutrophils is sufficiently large, a collection of pus forms. |
5) Phagocytosis of Pathogens by Neutrophils
The overall response to a splinter is depicted in the illustration below on the left and the short video animation on the right.:
Signs of Inflammation
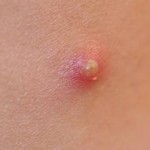 Some of the chemical messengers that are released during an inflammatory response dilate blood vessels and increase blood flow in the area of infection. The combination of increased blood flow and movement of white blood cells and fluid from blood into the tissues cause local redness and swelling, and the release of prostaglandins, histamine and other chemical signals caused localized tenderness and pain. Together, these produce the classic signs of inflammation:
Some of the chemical messengers that are released during an inflammatory response dilate blood vessels and increase blood flow in the area of infection. The combination of increased blood flow and movement of white blood cells and fluid from blood into the tissues cause local redness and swelling, and the release of prostaglandins, histamine and other chemical signals caused localized tenderness and pain. Together, these produce the classic signs of inflammation:
- redness (from increased blood flow)
- temperature increase (the affected area may be warm to touch due to increased blood flow)
- swelling (from movement of leukocytes and edema fluid into the tissue)
- pain & tenderness (from release of prostaglandins, histamine and other mediators)
The pimple shown on the right is a good example of a very localized inflammatory response, and it illustrates these characteristics. Note also that the collection of dead neutrophils is producing a whitish pustule in the center of the affected area.
The Complement System
The complement system consists of about 20 interacting proteins that greatly enhance the ability of phagocytic cells to identify and eliminate pathogens. The complement proteins are synthesized in the liver, and they circulate in blood in an inactive form. As part of the inflammatory response described above, gaps between endothelial cells allow leukocytes, fluid and proteins (including complement proteins) in blood to enter the inflamed tissue. Complement proteins coming into contact with PAMPs at the site of infection become activated, and they, in turn, activate more and more complement proteins i the proteins become activated, and the remain inactive until they are triggered by contact with PAMPs, but when the system is activated the proteins activate one another in sequence, & each step an what has been described as an amplification cascade.
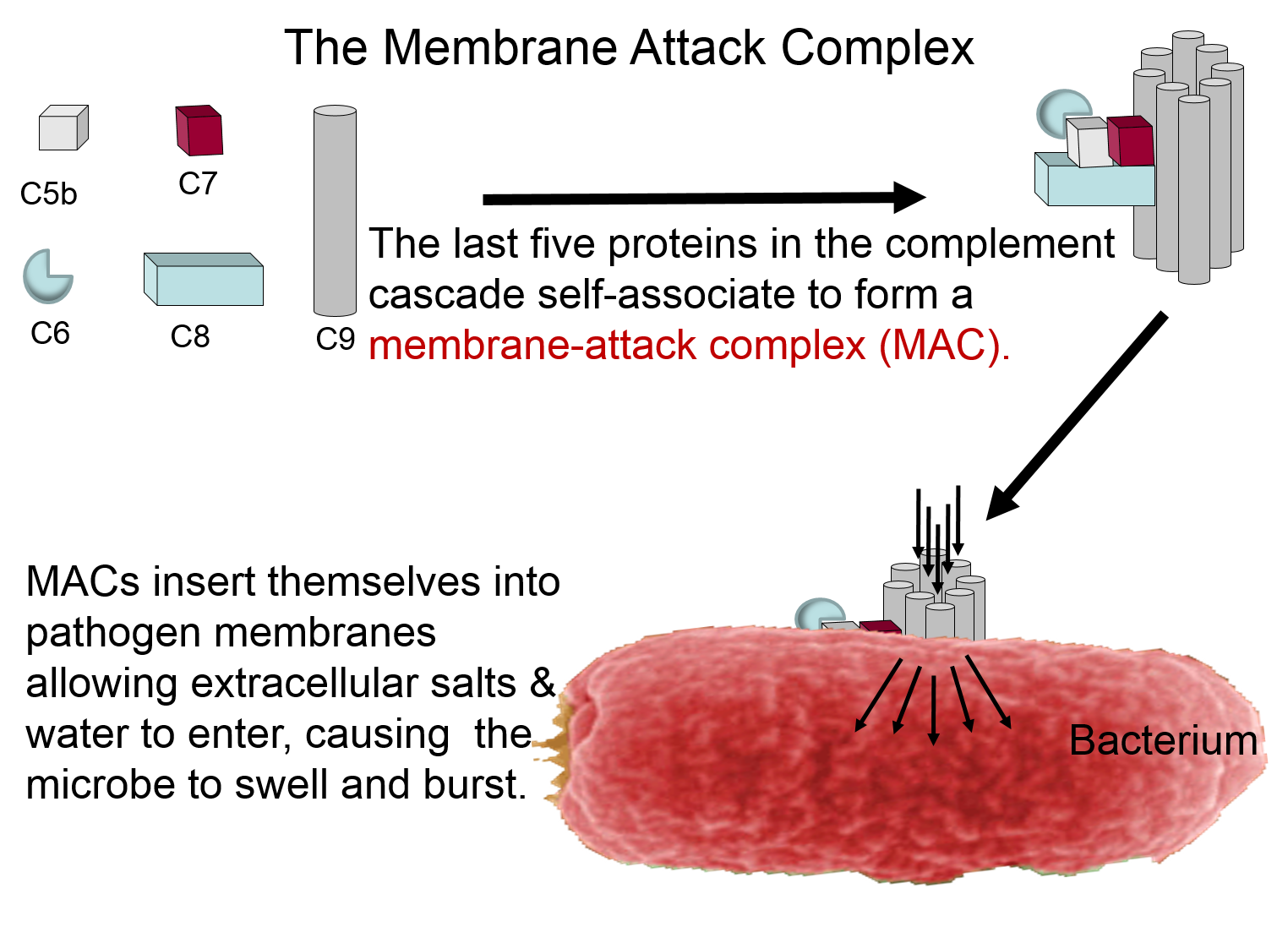
Membrane Attack Complex
The complement proteins contribute to the innate immune response by both destroying pathogens and by tagging them so that they can be more easily identified and destroyed by leukocytes. These functions are illustrated in the two panels below. The panel on the left shows how five of the complement proteins self-associate into a membrane-attack complex (MAC) when they become activated. The MAC inserts itself across the cell membrane of pathogens, creating a conduit through which ions and fluid can rush into the bacterium causing it to swell and burst.
Other Functions of the Complement Proteins
The next figure summarizes all of the functions of complement proteins. The MAC can cause lysis of bacteria, but complement proteins also enhance the inflammatory response and facilitate the action of antibodies. Their functions are:
- They can bind directly to the PAMPs on pathogens, and this tags them in a way that facilitates the identification and elimination of pathogens by phagocytic leukocytes.
- They trigger the release of histamine from mast cells.
- They facilitate the removal of antigen-antibody complexes. Antibodies are generated by the adaptive immune system, and, like complement proteins, they tag foreign substances to enhance their removal by phagocytic cells. However, unlike complement, antibodies are highly specific in their ability to identify pathogens and foreign substances.
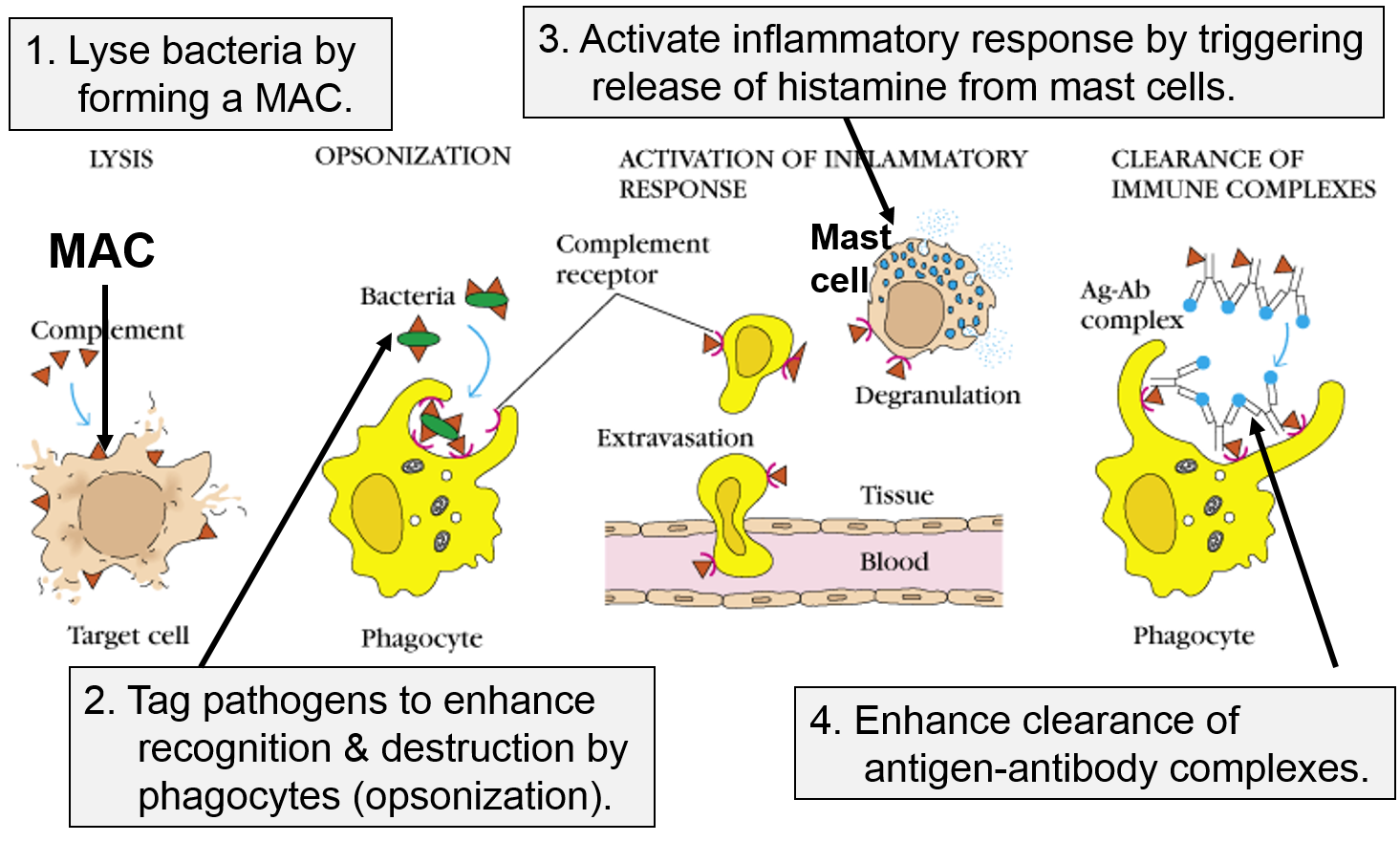
Image adapted from http://biosiva.50webs.org/complements.htm
Other Functions of the Complement Proteins
The panel on the right shows the MAC, but it also shows three other functions of complement proteins that enhance the inflammatory response.
- They can bind directly to the PAMPs on pathogens, and this tags them in a way that facilitates the identification and elimination of pathogens by phagocytic leukocytes.
- They trigger the release of histamine from mast cells.
- They facilitate the removal of antigen-antibody complexes. Antibodies are generated by the adaptive immune system, and, like complement proteins, they tag foreign substances to enhance their removal by phagocytic cells. However, unlike complement, antibodies are highly specific in their ability to identify pathogens and foreign substances.
Natural Killer Cells (NK Cells)
The PAMPs on the surface of bacteria and parasites are not present on the surface of viruses, but the innate immune system provides a means of defending against viral infection. .destroying our cells if they become infected with virus.
Vertebrates have "histocompatibility molecules," referred to as "major histocompatibility complex" molecules (MHC). Theses are large glycoprotein molecules that are found in the cell membranes of most vertebrate cells. In humans, the MHC molecules (also referred to as MHC antigens) are called Human Leukocyte Antigens (HLA). The MHC molecules play an important role in helping our immune cells to distinguish between our own cells (self) and foreign cells or substances (non-self). The degree of similarity in HLA antigens is a major factor in determining whether organ or stem cell transplantations will be successful. If a donor and recipient have similar HLA, the probability of success is much higher, and this is the basis on which the term "histocompatibility molecules" came into use. Prior to transplantation the laboratory will perform "tissue typing" in order to find a closely matching donor, i.e., one who has a similar set of HLA. on their cell membranes. In humans they are called the human leukocyte antigen system (HLA).
All of our nucleated cells (not red blood cells or platelets) have MHC class I molecules on their surface. However, if our cells become infected with virus, the expression of MHC class I molecules diminishes. Natural killer cells (NK cells) provide a means of monitoring our cells through a dual mechanism for binding to them, as illustrating in the image below. The normal cell on the left has MHC class I molecules on its surface, allowing both binding sites to be occupied. In essence, the presence of the MHC class I prevents the NK cell from attacking it. However, the cell on the right is missing MHC class I molecules, and the NK cell is stimulated to release substances (perforin and granzymes) that create holes in the cell's membrane that cause the cell to burst as ions and water flow into it. By killing the virus infected cell in this way, the production of more virus particles is terminated. Note that some cancers also diminish the expression of MHC class I molecules, and there is evidence that NK cells sometimes eliminate cells that have become cancerous through this mechanism.
Note also that although NK cells are lymphocytes, they are considered to be part of the innate immune system, because their ability to eliminate damaged cells is non-specific, i.e., it is not triggered by recognition of a specific foreign antigen.
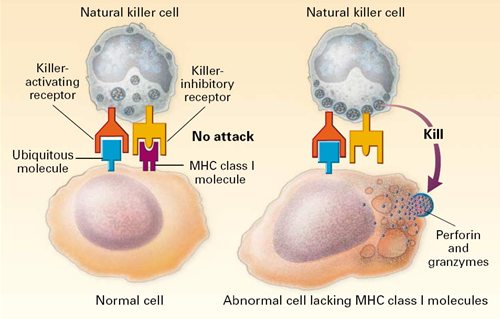
Image source: http://highscope.ch.ntu.edu.tw/wordpress/?p=536