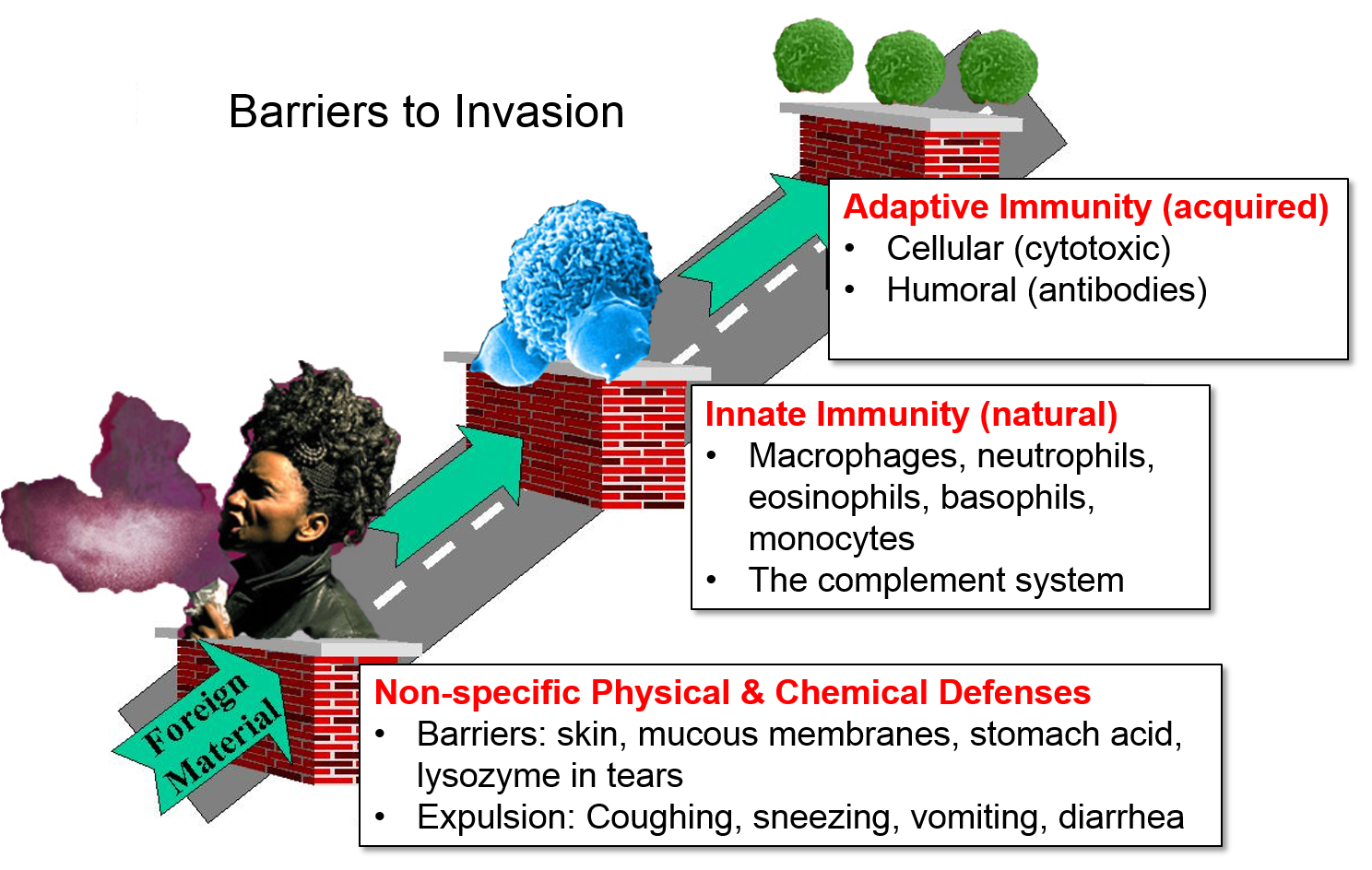
Defense Mechanisms
There are several simple physical and chemical barriers that constitute and important first line of defense.
Our skin provides a highly effective barrier to infectious agents despite the fact that skin is colonized by an impressive array of microbial agents. Injury to the skin (abrasions, cuts, incisions, burns, etc.) or penetration.(insect bites, splinters, needle sticks, stabs) can, of course, breach the barrier and provide a portal of entry for infectious agents.
Given the effectiveness of intact skin, our major vulnerabilities are:
These are our major vulnerabilities, but we have evolved non-specific defenses for these.
The video below shows cells lining the bronchial portion of respiratory tract. The cells have hair-like projections (cilia) that beat rhythmically. The coordinated beating sweeps dust and pathogens trapped in the mucus layer back up the respiratory tree to the pharynx, where it can be either swallowed or expectorated.
Source: http://www.youtube.com/watch?v=1yEVrJxQTV0
These non-specific barriers are extremely important, but they represent just the first line of defense. The innate and adaptive immune systems provide additional important barriers to infection.

These non-specific barriers are extremely important, but they represent just the first line of defense. The innate and adaptive immune systems provide additional important barriers to infection.
From a functional perspective, the immune system consists of innate immunity and adaptive immunity, two separate, but interacting and overlapping defensive systems that provide an additional array of defensive weapons. In addition, innate immunity and adaptive immunity are activated by recognition of molecular shapes that are "foreign" to our body. By distinguishing between "self" and "non-self" these systems are (normally) able to identify, destroy, and remove foreign cells, infectious agents, and large foreign molecules without directly attacking our own cells and tissues. [Note, however, that dysfunctional activation of our immune system sometimes has very harmful effects. See later sections on Recognition of "Self," Autoimmune Disease, and Cytokine Storm.]
Many bacteria, viruses, and protozoa have glycoproteins and glycolipids on their surface that have distinctive shapes (referred to as "pathogen-associated molecular patterns" or PAMPS) on their surface that enable them to be recognized in a non-specific way as "non-self" by the innate immune system. There are perhaps 100-200 of these PAMPs that have remained unchanged over the course of evolution, and they are molecular shapes that are not present in our tissues. The innate immune system has certain "sentinel cells (monocytes, macrophages, and specialized macrophages called a dendritic cells) that have so-called toll-like receptors that bind to PAMPs, triggering rapid cellular responses directed against the pathogens. The responses include:
It is possible for a given PAMP to be present on a number of different types of pathogen, and the innate system will respond to them in the same way without distinguishing among them. Consequently, the innate system is non-specific in how it recognizes and responds to pathogens. And this system is referred to as innate or natural immunity, because the sentinel cells in the innate system will recognize a PAMP and respond to it on the first encounter.
These responses will be described in greater detail later in this module.
Cells of the lymphatic (or lymphoid) system provide adaptive immunity, which, unlike innate immunity, is highly specific in its ability to recognize and defend against specific foreign agents using both cellular weapons (e.g., cytotoxic T-lymphocytes) and humoral weapons (antibodies manufactured by plasma cells). The lymphatic system is distinct from the arterial and venous systems, but like them, it consists of a complex network of vessels (lymphatic ducts), and the distribution of the lymphatic network often runs in parallel with the arterial and venous systems. Along the lymphatic vessels, there are intermittent lymph nodes, which filter lymph and also house many defensive cells (leukocytes or "white blood cells") and provide a site where the various leukocytes can communicate with one another. When fighting an infection, nearby lymph nodes often become enlarged due to aggregation and increased production of leukocytes and and removal of foreign material. Filtered lymph eventually is emptied into the subclavian vein where it mixes with blood and contributes to the plasma fraction of blood. The thymus and the spleen are also important components of the lymphatic system. The lymphatic system, thymus, and spleen play important roles in immune function, but cellular elements of the immune system are the real "soldiers" in the battle against foreign agents.
Source: http://www.frequencyrising.com/immune_system.htm)
The illustration below shows the derivation of the key cells involved in the innate and adaptive immune systems.
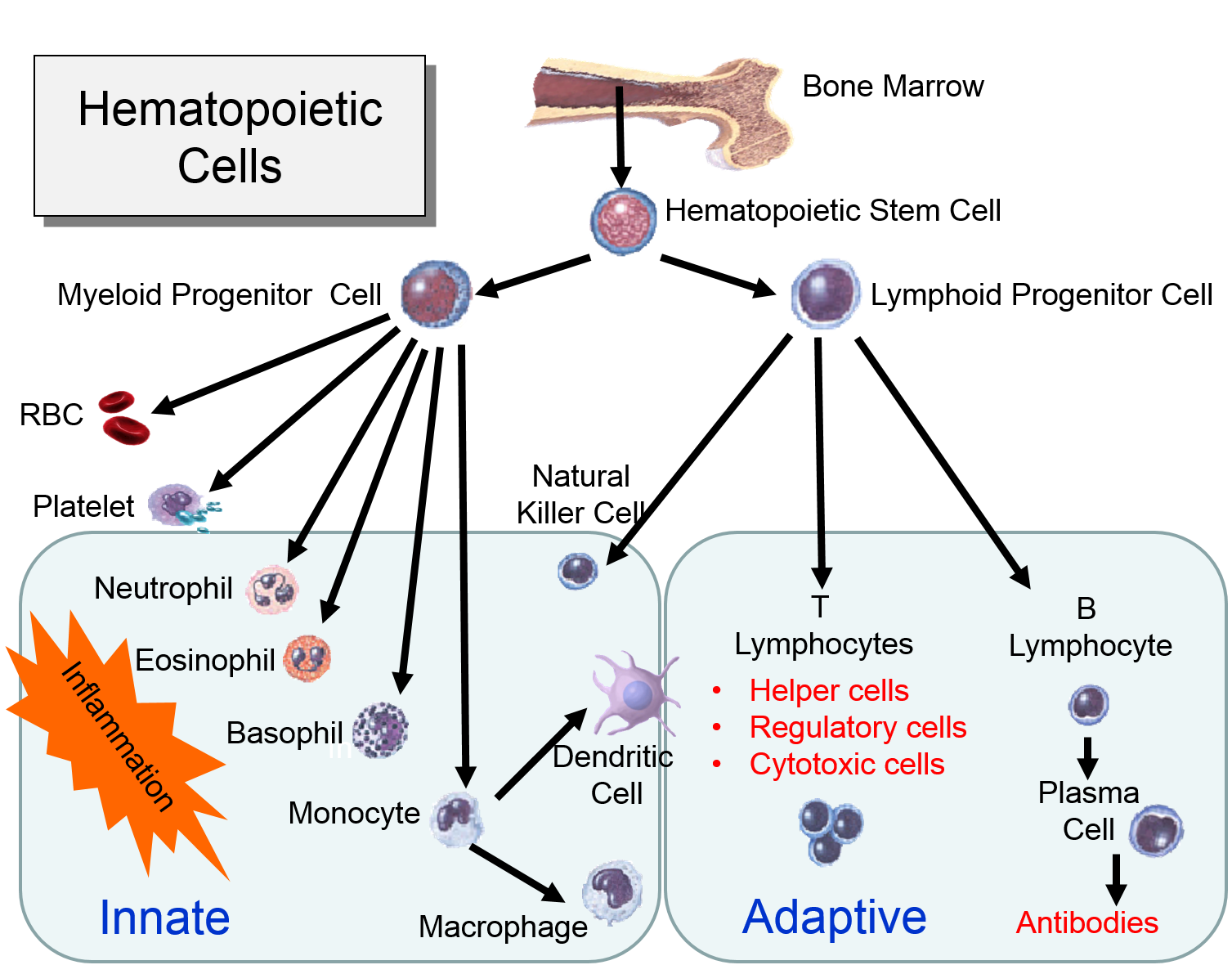
Myeloid Cells of the Innate Immune System
(Point your cursor at each tab to see a brief description of each cell type.
Lymphoid Cells of the Adaptive Immune System)
The adaptive system differs from the innate system in several ways:
Injury or infection will an inflammatory response, which is an array of cellular events that is a major component of the innate immune response. To illustrate, consider the events that would be expected to take place after a splinter of wood sticks into your finger or you cut your foot on a piece of glass while walking barefooted. In both cases, the barrier function of the skin is breached, tissues are injured, and bacteria are introduced into the underlying tissues.
1) Binding of PAMPs to Toll-like Receptors
Injury to cells will prompt them to release chemical messengers, such as prostaglandins. In addition, the exterior surfaces of pathogens often have PAMPs on glycoproteins projecting from theirl surface or the flagella of motile pathogens. These PAMPs bind to toll-like receptors on macrophages, neutrophils, and dendritic cells, and they are also present on epithelial cells in the respiratory and gastrointestinal tracts. Binding of PAMPs to toll-like receptors in tissues is the alarm signal that triggers an inflammatory response, and PAMPs can also activate the complement system (which will be described later in this module.) Neutrophils are not normally present in tissues, unless there is injury or infection, but neutrophils constantly circulate in blood in large numbers. Nevertheless, macrophages are present in tissues, and always ready to respond.
2) Phagocytosis of Pathogens by Macrophages
Binding of a pathogen's PAMPs to the toll-like receptors on tissue macrophages activates them and initiates phagocytosis of the pathogen. The sequence of drawings below illustrate the steps in phagocytosis as a bacterium binds to toll-like receptors and is then engulfed and digested.
|
A bacterium (red) binds to a toll-like receptor on a macrophage. |
The cell begins to flow around the bacterium, gradually engulfing it. |
A portion of the cell membrane pinches off forming a phagosome. |
The phagosome fuses with a lysosome which contains digestive enzymes. |
The enzymes break the pathogen & receptor into pieces, & some antigen fragments are bound to antigen-presenting MHC II molecules. |
The residue of the bacterium is expelled by exocytosis, & the antigen-bearing MHC II molecule is inserted into the cell membrane. |
Note also that some of the molecular fragments of the bacterium are combined with newly-synthesized MHC Class II molecules in the endoplasmic reticulum. This complex is then inserted in the macrophage's cell membrane, effectively "displaying" the pathogenic antigens on its surface. This is an important mechanism by which macrophages alert the adaptive immune system to the presence of a foreign agent. This will be described in the section on adaptive immunity.
3) Signaling to Other Cells
Biochemical signals are released from injured cells (e.g., prostaglandins) and mast cells (e.g., histamine) and from macrophages. Activation of macrophages by binding of PAMPs stimulates the
synthesis and release of a variety of cytokines and chemokines.
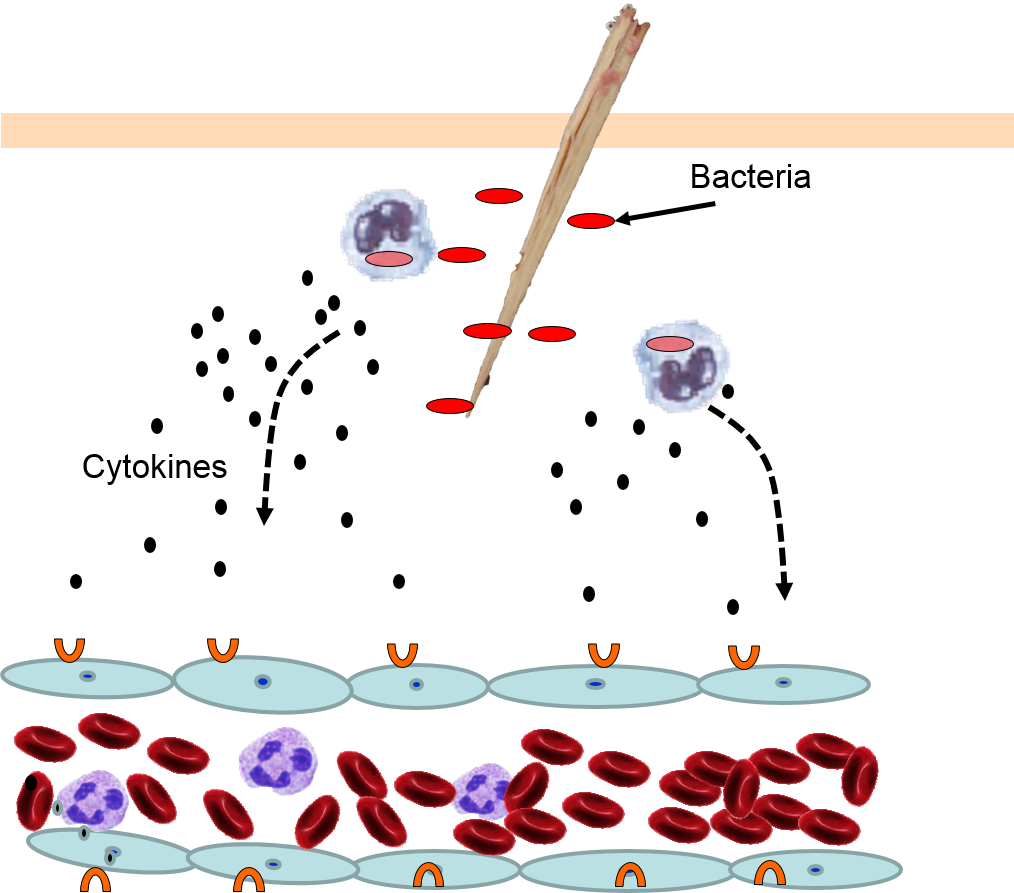
4) Changes in Local Blood Vessels
The various signaling molecules that are released after injury or infection (prostaglandins, histamine, and cytokines) induce important changes in local capillaries. Cytokines diffuse to local capillaries and bind to receptors which induce changes in the endothelial cells lining local capillaries, as depicted below.
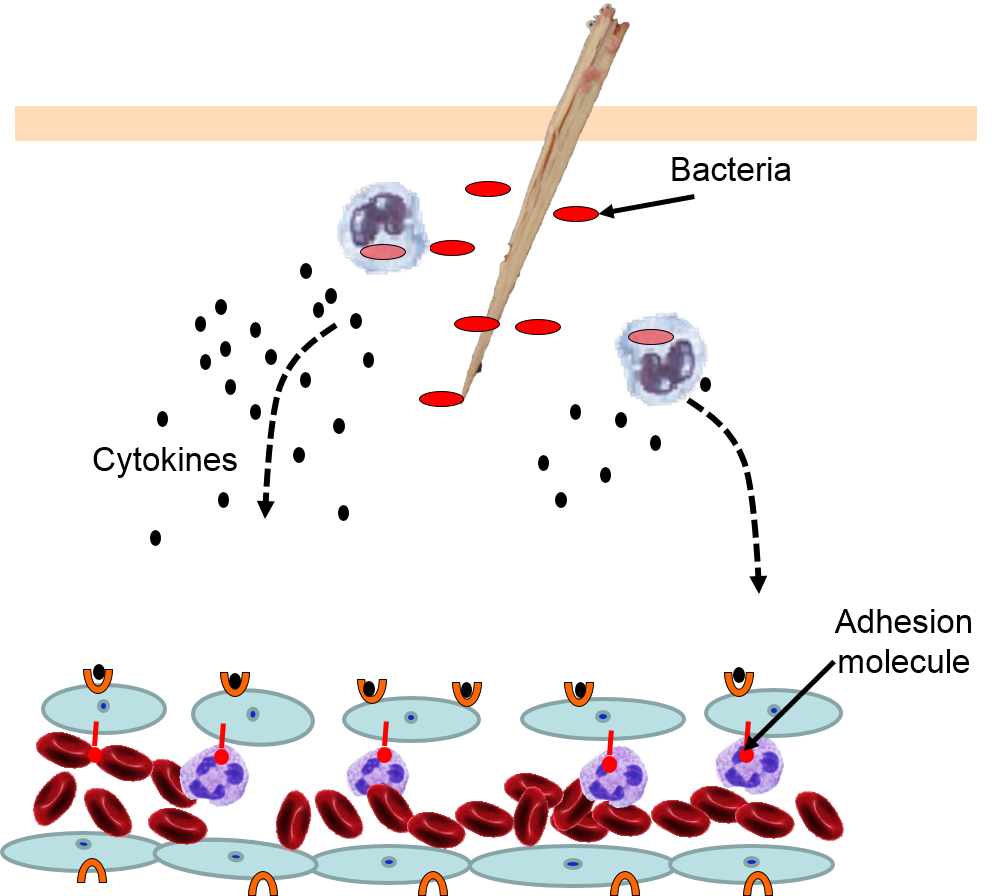
Cytokines bind to receptors (orange semi-circles) on endothelial cells of a nearby capillary. This causes expression of adhesion molecules on the luminal surface of the endothelial cells (red lollipops), and causes endothelial cells to change shape, creating gaps between them.
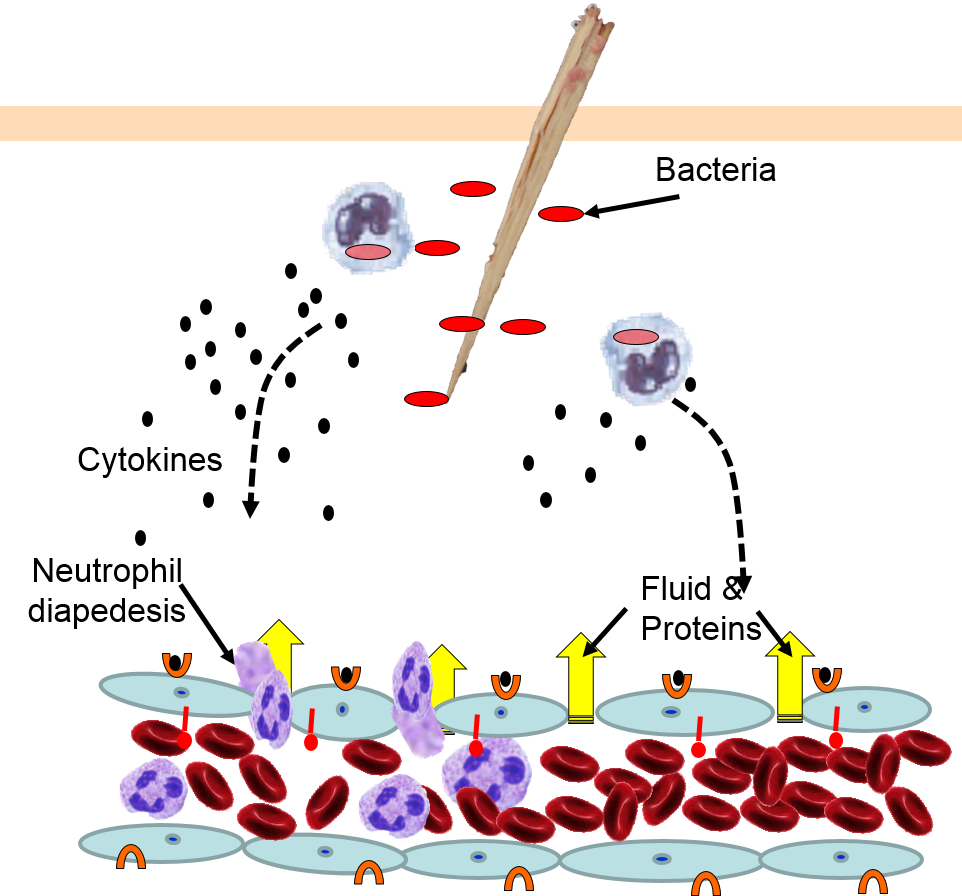
Neutrophils circulating in blood briefly bind to the adhesion molecules and then migrate between the endothelial cells (diapedesis) and following the trail of chemokines (not shown) toward the site of infection. The gaps in the endothelium also allow fluid and proteins from blood to enter the tissue.
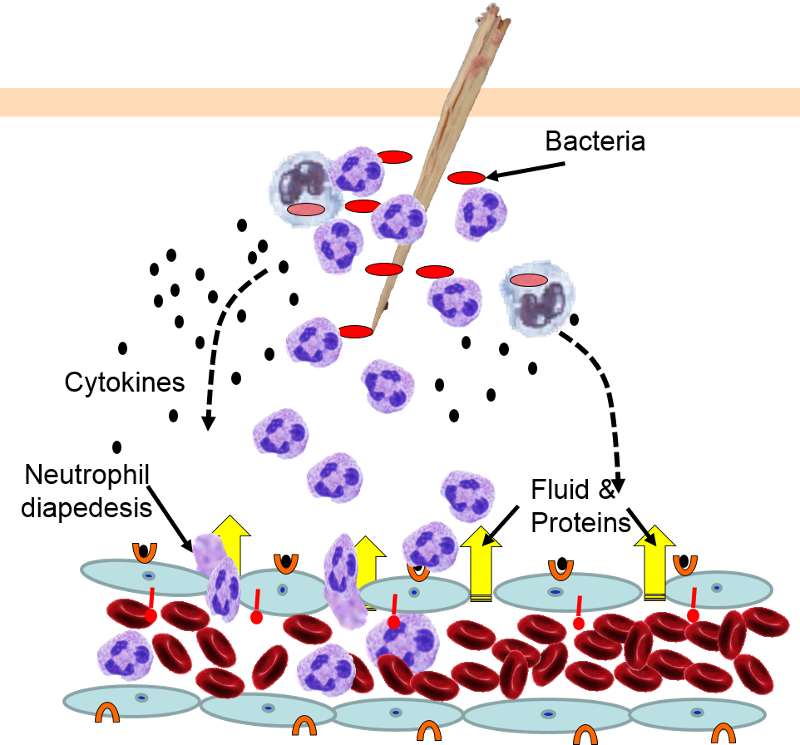
Neutrophils kill the invading bacteria by phagocytosis aided by complement proteins which tag the bacteria to facilitate identification and phagocytosis of the pathogens. After phagocytosing bacteria, the neutrophils die. If the number of dead neutrophils is sufficiently large, a collection of pus forms.
5) Phagocytosis of Pathogens by Neutrophils
The overall response to a splinter is depicted in the illustration below. A splinter has pierced the skin and triggered an inflammatory response.
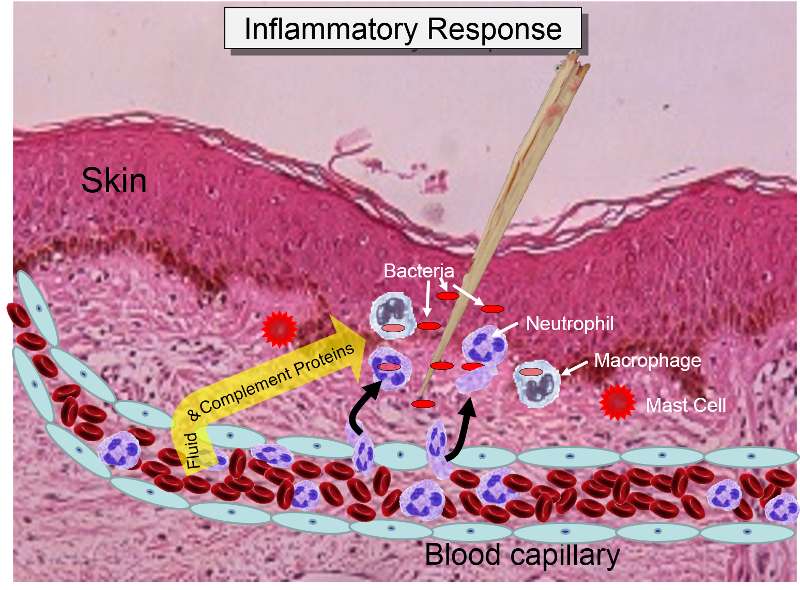
Some of the chemical messengers that are released during an inflammatory response dilate blood vessels and increase blood flow in the area of infection. The combination of increased blood flow and movement of white blood cells and fluid from blood into the tissues cause local redness and swelling, and the release of prostaglandins, histamine and other chemical signals caused localized tenderness and pain. Together, these produce the classic signs of inflammation:
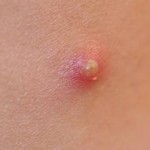
The pimple shown below is a good example of a very localized inflammatory response, and it illustrates these characteristics. Note also that the collection of dead neutrophils is producing a whitish pustule in the center of the affected area.
The complement system consists of about 20 interacting proteins that greatly enhance the ability of phagocytic cells to identify and eliminate pathogens. The complement proteins are synthesized in the liver, and they circulate in blood in an inactive form. As part of the inflammatory response described above, gaps between endothelial cells allow leukocytes, fluid and proteins (including complement proteins) in blood to enter the inflamed tissue. Complement proteins coming into contact with PAMPs at the site of infection become activated, and they, in turn, activate more and more complement proteins i the proteins become activated, and the remain inactive until they are triggered by contact with PAMPs, but when the system is activated the proteins activate one another in sequence, & each step an what has been described as an amplification cascade.
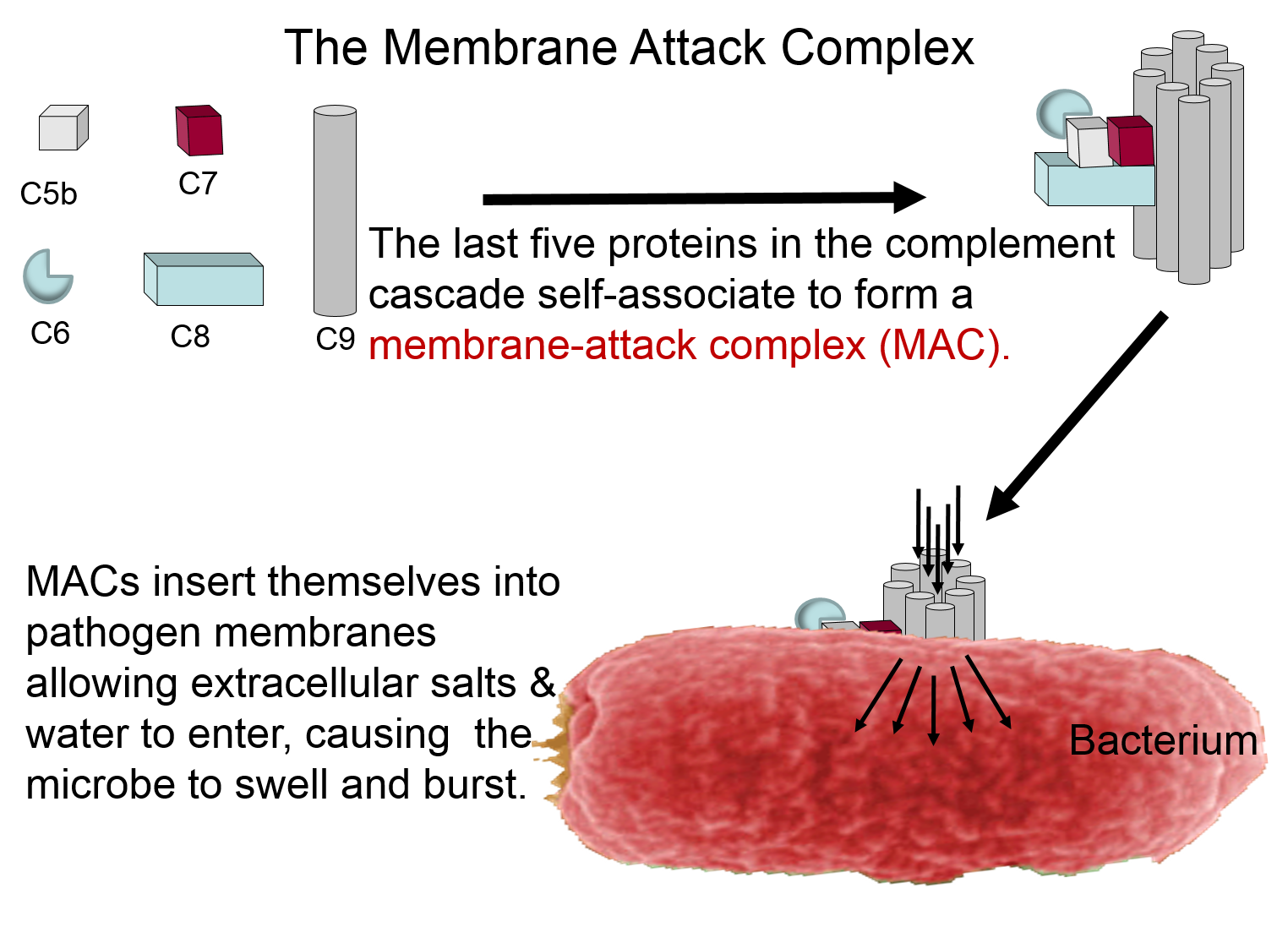
Membrane Attack Complex
The complement proteins contribute to the innate immune response by both destroying pathogens and by tagging them so that they can be more easily identified and destroyed by leukocytes. The panel below shows how five of the complement proteins self-associate into a membrane-attack complex (MAC) when they become activated. The MAC inserts itself across the cell membrane of pathogens, creating a conduit through which ions and fluid can rush into the bacterium causing it to swell and burst.
Other Functions of the Complement Proteins
The next figure summarizes all of the functions of complement proteins. The MAC can cause lysis of bacteria, but complement proteins also enhance the inflammatory response and facilitate the action of antibodies. Their functions are:
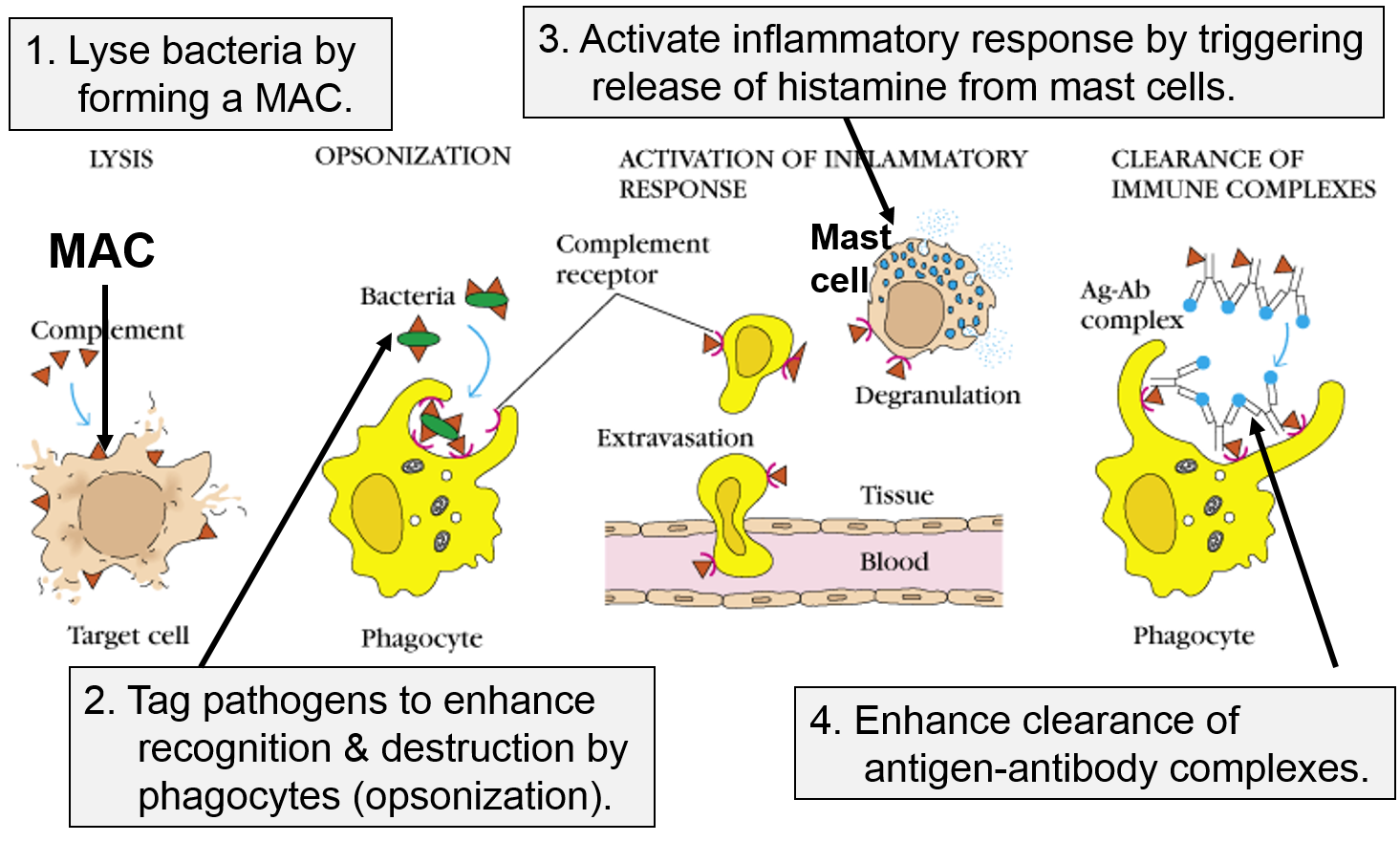
Image adapted from http://biosiva.50webs.org/complements.htm
The PAMPs on the surface of bacteria and parasites are not present on the surface of viruses, but the innate immune system provides a means of defending against viral infection. .destroying our cells if they become infected with virus.
Vertebrates have "histocompatibility molecules," referred to as "major histocompatibility complex" molecules (MHC). Theses are large glycoprotein molecules that are found in the cell membranes of most vertebrate cells. In humans, the MHC molecules (also referred to as MHC antigens) are called Human Leukocyte Antigens (HLA). The MHC molecules play an important role in helping our immune cells to distinguish between our own cells (self) and foreign cells or substances (non-self). The degree of similarity in HLA antigens is a major factor in determining whether organ or stem cell transplantations will be successful. If a donor and recipient have similar HLA, the probability of success is much higher, and this is the basis on which the term "histocompatibility molecules" came into use. Prior to transplantation the laboratory will perform "tissue typing" in order to find a closely matching donor, i.e., one who has a similar set of HLA. on their cell membranes. In humans they are called the human leukocyte antigen system (HLA).
All of our nucleated cells (not red blood cells or platelets) have MHC class I molecules on their surface. However, if our cells become infected with virus, the expression of MHC class I molecules diminishes. Natural killer cells (NK cells) provide a means of monitoring our cells through a dual mehanism for binding to them, as illustrating in the image below. The normal cell on the left has MHC class I molecules on its surface, allowing both binding sites to be occupied. In essence, the presence of the MHC class I prevents the NK cell from attacking it. However, the cell on the right is missing MHC class I molecules, and the NK cell is stimulated to release substances (perforin and granzymes) that create holes in the cell's membrane that cause the cell to burst as ions and water flow into it. By killing the virus infected cell in this way, the production of more virus particles is terminated. Note that some cancers also diminish the expression of MHC class I molecules, and there is evidence that NK cells sometimes eliminate cells that have become cancerous through this mechanism.
Note also that although NK cells are lymphocytes, they are considered to be part of the innate immune system, because their ability to eliminate damaged cells is non-specific, i.e., it is not triggered by recognition of a specific foreign antigen.
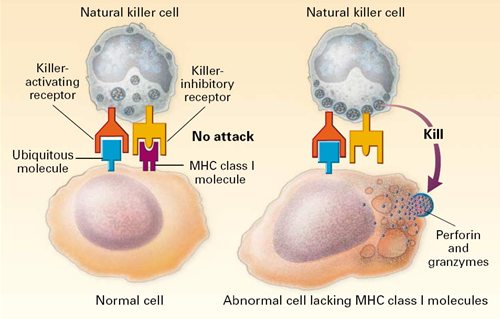
Image source: http://highscope.ch.ntu.edu.tw/wordpress/?p=536
The MHC molecules also play a major role in directing the adaptive immune system. There are two major classes of MHC molecules: MHC class I and MHC class II.
MHC I glycoproteins are present on all of the nucleated cells in the body (they are not present on red blood cells or platelets). The function of MHC class I molecules is to take pieces of any protein synthesized within the cell and "present" them on the cell surface. Cells are constantly turning over cell proteins, removing old ones and replacing them with new ones. As part of this process, recycled proteins are broken into small fragments called peptides, and these are sent to the endoplasmic reticulum where some of the peptide fragments bind to a groove on the surface of newly-synthesized MHC class I molecules. The MHC-peptide complex is then transported to the cell surface and inserted into the cell membrane so that the peptide fragment is "presented" to the exterior of the cell where it is accessible to lymphocytes.
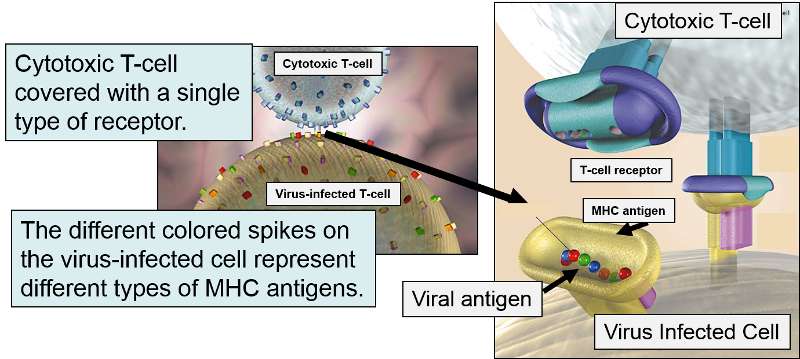
This mechanism becomes extremely valuable if a cell becomes infected with a virus or if it undergoes malignant transformation (becomes cancerous). Viruses are not able to reproduce on their own; they must use a host cell's synthetic "machinery" to make copies of the viral components, including viral proteins. Some of these viral proteins will also be broken into peptide fragments and combined with MHC class I molecules on the cell surface.
If a cytotoxic T-cell (CD+8) encounters a peptide fragment that has a complementary shape to its receptor, it will bind to the MHC-peptide complex and secrete cytotoxic molecules that penetrate the infected cell and kill it, effectively ending the production of more virus particles. Consequently, MHC Class I proteins work to present the types of proteins being synthesized within a cell, so that they can be monitored by lymphocytes in order to destroy cells producing unfamiliar proteins, i.e., cancer cells or virus-infected cells.
MHC II glycoproteins are only present on macrophages, dendritic cells, and B cells. All three of these cell types are capable of phagocytosis, and their function is to engulf antigens that originate from outside the cell, e.g., on bacteria. After the exogenous antigens are broken down, the resulting peptide fragments are bound to MHC II molecules and presented on the cell surface.
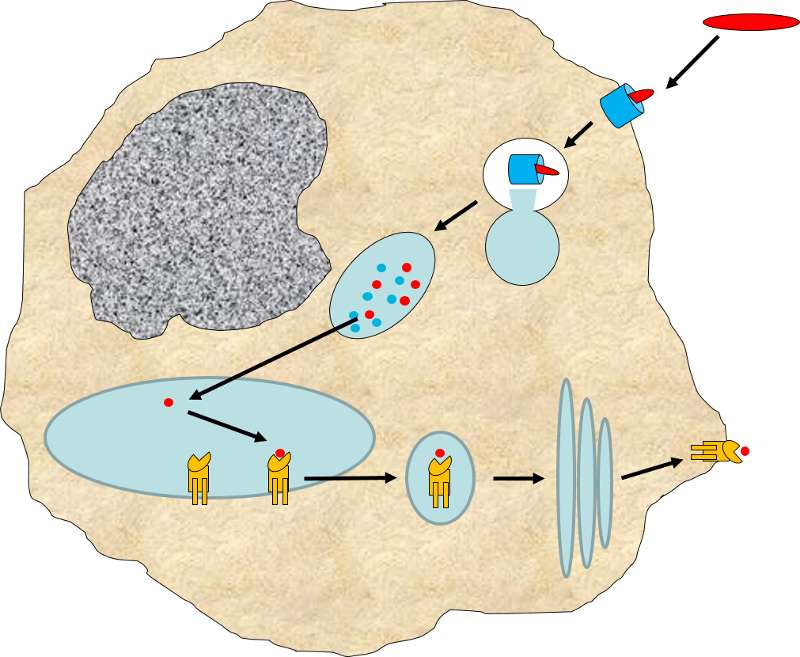
These cells will typically migrate to nearby lymph nodes where helper T cells with receptors that match the antigen have a greater opportunity to encounter the antigen and bind to it.
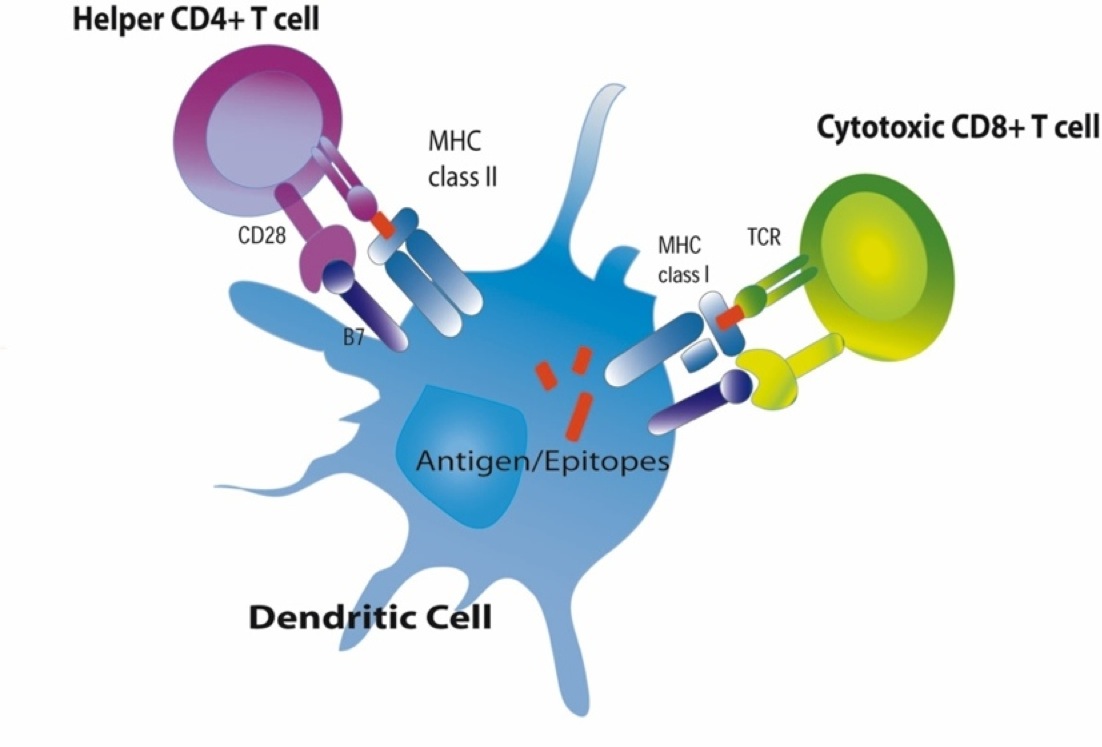
When this occurs, the helper T cell lymphocytes become activated and begin to release cytokines that attract other cells to the area of infection in order to destroy the infectious agents with that antigenic material. B lymphocytes can also engulf foreign antigens, break them down, and display the resulting peptides on MHC II molecules on their surface. If a helper T lymphocyte binds to a peptide fragment on the surface of a B cell, it stimulates the B cell to divide repeatedly and differentiate into plasma cells which produce antibody against the antigenic material.
The video below illustrates the role of macrophages in presenting antigen.
The innate immune system is triggered by PAMPs or, in the case of natural killer cells, by the absence of MHC class I molecules on a cell's surface, but the adaptive immune system is triggered by very specific molecular shapes, which are generally referred to as antigens. The next image is a representation of an influenza virus, which consists of an exterior shell of hemagglutinen and neuraminidase proteins and eight RNA strands in its core.
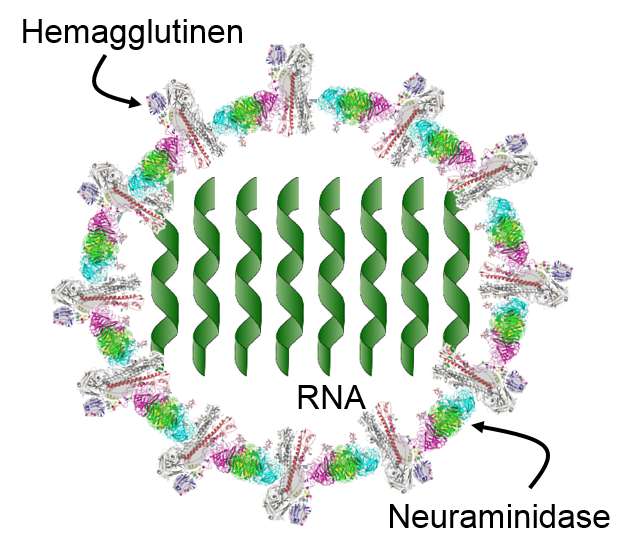
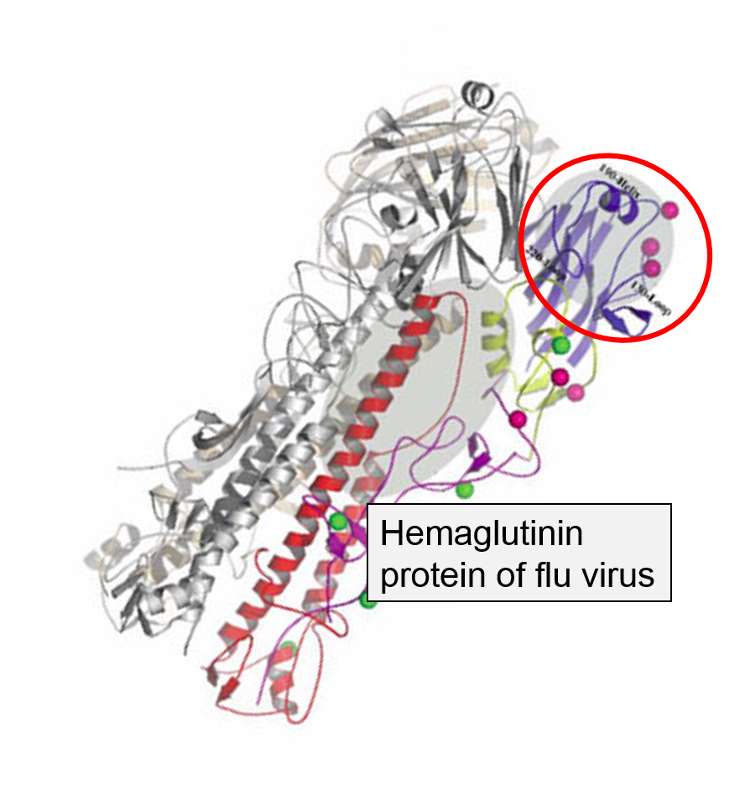
The hemagglutinen and neuraminidase proteins are potential antigens, but there are only specific portions of these molecules that might be "recognized" by our immune system. The illustration below is an enlarged image of a hemagglutinen protein, and the portion of the molecule circled in red might represent a specific shape, i.e., an epitope, on the hemagglutinen molecule that would be recognized by our immune system.
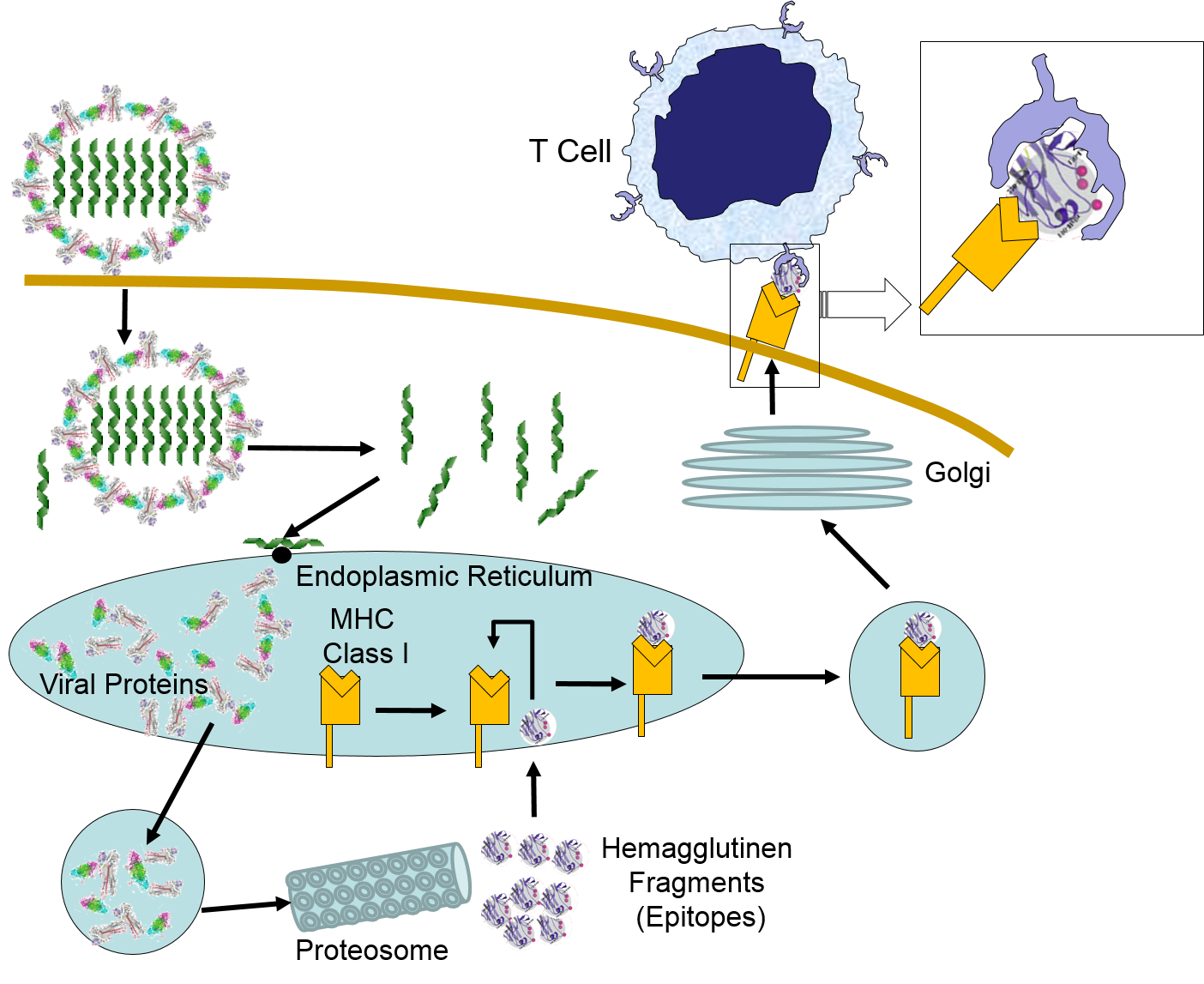
When influenza virus infects our cells (e.g., epithelial cells in our nose and throat) its protein coat disintegrates, and the viral RNA uses our ribosomes and substrates to produce more viral proteins and more copies of its DNA. However, as noted above, samples of internally synthesized proteins (including viral proteins) are broken down in proteosomes, and the fragments are complexed with MHC Class I molecules in the endoplasmic reticulum. The MHC Class I and attached fragments are then inserted into the cell membrane where the fragments are "presented" to cells of the immune system. These events are depicted in the figure below. Helper T cells with matching receptors would become activated and recruit additional lymphocytes, and cytotoxic T cells with matching receptors would bind to the cell and secrete cytotoxic molecules that penetrate the infected cell and kill it, effectively ending the production of more virus particles.
Virus binds to a human epithelial cell and becomes internalized. It then sheds its protein coat and begins to replicate viral RNA and proteins uses the cells organelles and substrates. Some of the viral proteins are transported from the endoplasmic reticulum to proteosomes which break them into fragments which are bound to MHC Class I molecules. These are then transported to the cell membrane and inserted with the protein fragments "presented" to the exterior of the cell where T cells with matching receptors can bind to the fragments and become activated.

B lymphocytes can become activated by direct contact with a pathogen or foreign protein if they have a receptor that is complementary to an epitope on the foreign agent. Helper T cells that have become activated by antigen presentation will further stimulate the activated B cell to replicate over and over and to transform into a large clone of plasma cells that produce antibodies specific for that epitope. These antibodies are widely distributed in the circulation and can bind to the epitopes, tagging the foreign agents to facilitate its identification and destruction by phagocytic cells. The image below shows an antibody binding to a specific epitope on two virus particles. Keep in mind, however, that antibodies can similarly participate in defense against any agent or substance that has matching epitopes.
What Antibodies Do
Classes of Antibodies
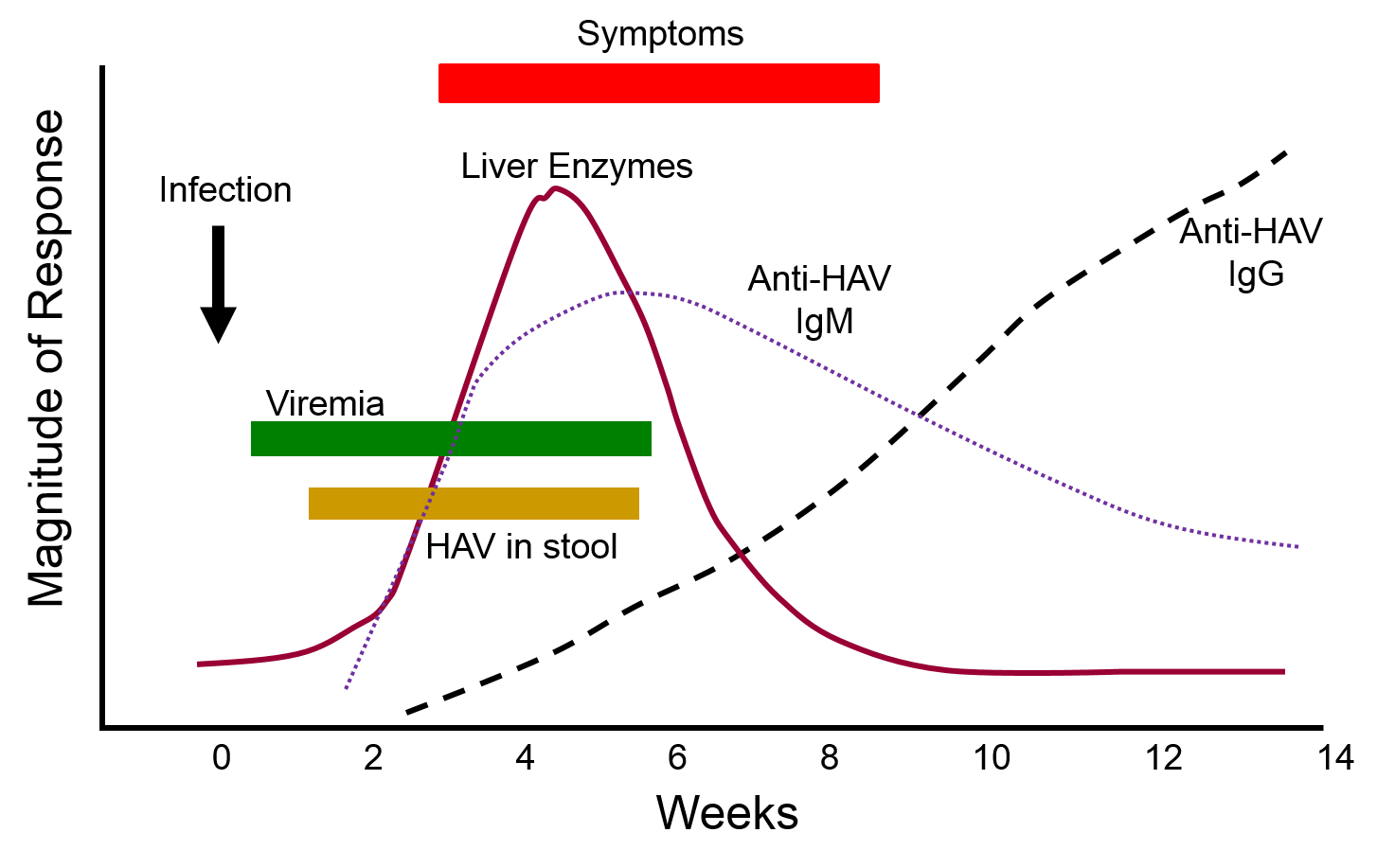
The graph below depicts the sequence of events that occur during infection with hepatitis A virus (HAV). Note, first, that the presence of virus in blood (viremia) and in stool occurs well before the onset of symptoms, making it easy for a victim to transmit the virus to others. Also, note that levels of IgM antibodies in blood rise early and then begin to decline. IgG levels rise somewhat later, but they persist for a much longer time. By measuring the titers (concentrations) of both IgM and IgG antibodies against HAV, it is possible to determine whether an individual was recently infected, or if they were infected some time ago. This information could be important in determining whether a particular food handler, for example, was responsible for an outbreak of hepatitis A.
 Immunization primes the adaptive immune system to produce an immune response without actually being infected. Weakened or killed pathogens or antigenic components of a pathogen are administered to evoke a primary immune response of the adaptive immune system. This initial exposure to the antigens of an infectious agent trigger a typical immune response. Most of the immune response rapidly diminishes after a vaccination, but some lymphocytes persist with an immunologic memory. As a result, if the same pathogen infects a vaccinated person at a later date, the memory cells rapidly spring into action and trigger a much more rapid adaptive immune response than occurred with the primary exposure.
Immunization primes the adaptive immune system to produce an immune response without actually being infected. Weakened or killed pathogens or antigenic components of a pathogen are administered to evoke a primary immune response of the adaptive immune system. This initial exposure to the antigens of an infectious agent trigger a typical immune response. Most of the immune response rapidly diminishes after a vaccination, but some lymphocytes persist with an immunologic memory. As a result, if the same pathogen infects a vaccinated person at a later date, the memory cells rapidly spring into action and trigger a much more rapid adaptive immune response than occurred with the primary exposure.
The Chinese used "variolation" - exposing uninfected individuals to matter from smallpox lesions – to prevent smallpox. Pus from a smallpox lesion could be placed under the skin with a needle. Dried, powdered scabs from smallpox lesions could be inhaled or placed in a vein with a needle.
Lady Mary Wortley Montagu, wife of the British Ambassador to Turkey, observed this method in the early 1700s and brought it back to England. Results ranged from a mild illness (most) to death (a few); mortality & morbidity from smallpox was certainly lower with variolation.
Edward Jenner was interested when a milkmaid told him she could not catch smallpox because she had had cowpox; he noted that many of the milk maids did not get smallpox. (They were renowned for their unblemished skin.) In 1796 he infected a young boy with cowpox, allowed him to fully recover, and then intentionally injected the boy with pus from a smallpox lesion. The boy did not become ill. Jenner published a book and people began intentionally infecting themselves with cowpox. It was called "vaccination," after "vacca," the Latin word for cow, and the substance used to vaccinate was called a "vaccine."
Most of the lymphocytes produced in a primary immune response are involved in fighting the pathogens, but as the clone of lymphocytes expands a few thousand of them differentiate into memory cells which persist for months or years. If the same pathogen invades the organism again, the memory cells will again bind to the pathogen and begin to replicate, but memory cells can replicate more quickly. As a result, a secondary exposure to a given antigen triggers an immune response that is much more rapid and more vigorous than that seen with the first exposure.
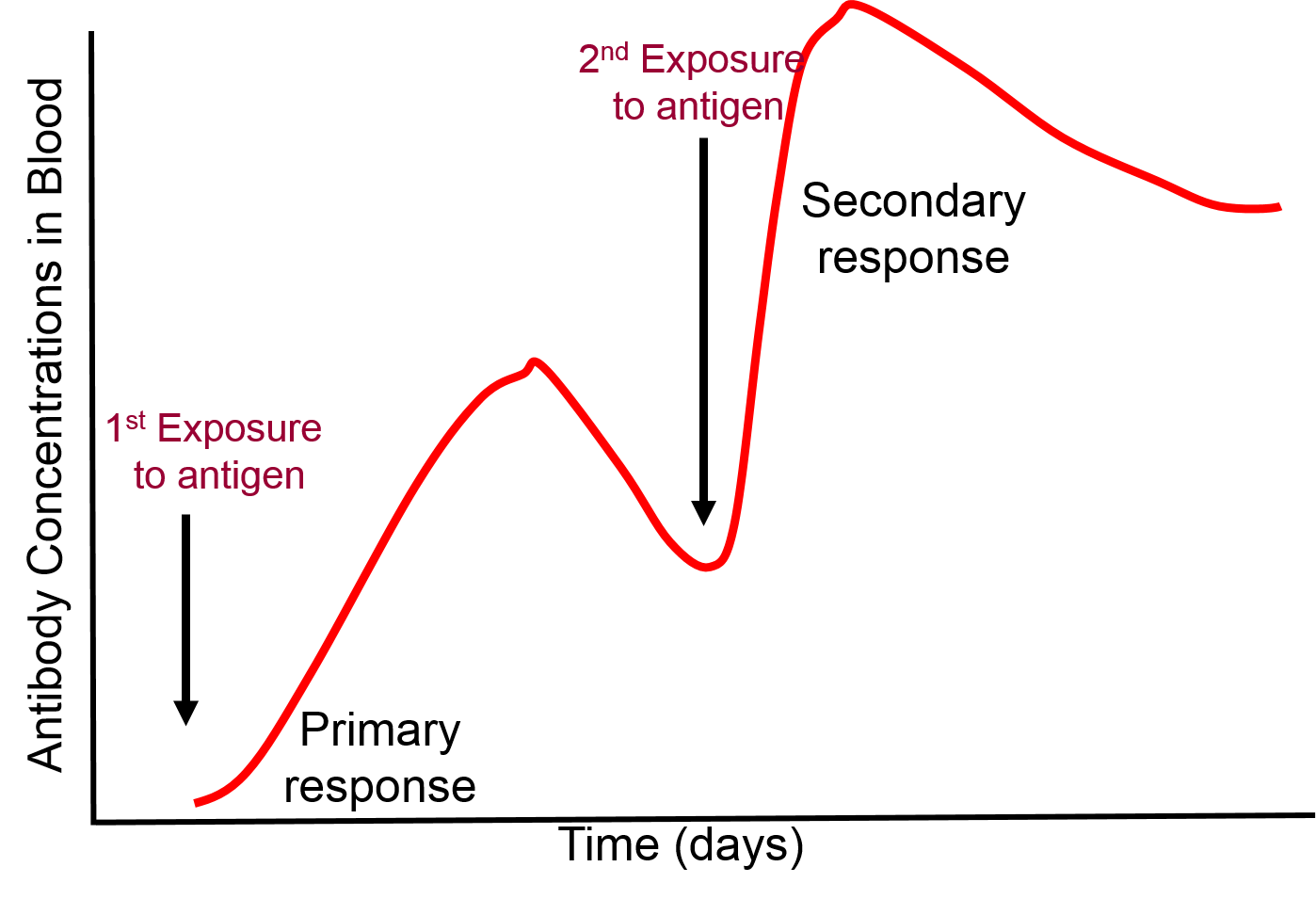 .
.
Active immunity occurs when an individual is infected with a pathogen or if they are vaccinated. Exposure to the pathogen's antigens by either of these will result in a primary immune response and immunologic memory. However, it is also possible in some circumstances to protect a susceptible person by giving them the antibodies produced by another person. For example, if we were to take serum from people who had previously been infected with hepatitis A virus (HAV), it would contain significant concentrations of IgG against HAV. It is possible to pool serum from previously infected individuals and then inject this immunoglobulin G into individuals who may have been recently been exposed to HAV in order to thwart the infection and prevent them from becoming a clinically active case.
In essence, passive immunization:gives antibodies made by others (e.g., pooled gamma globulin that will immediately recognize and neutralize an antigen to provide immediate protection. However, this passive form of protection bypasses the steps in primary exposure, and it does not produce immunologic memory. Moreover, the protection afforded by this passive form of immunity only lasts as long as the exogenous antibodies, about 3-4 months. After the exogenous antibodies disappear, the individual is just as susceptible as a person who had never been exposed.
|
CDC - Recommendations for Post-exposure Prophylaxis for Hepatitis A Virus
"Persons who have recently been exposed to HAV and who have not been vaccinated previously should be administered a single dose of single-antigen Hepatitis A vaccine or IG (0.02 mL/kg) as soon as possible, within 2 weeks after exposure. The guidelines vary by age and health status:
|
IgG is able to cross the placenta from mother to fetus. As a result, newborn infants receive some passive immunity from antigens to which their mother has been exposed. However, this passive protection disappears over a period of 3-4 months, so it is important for the infant to develop active immunity through vaccinations (or by being infected and developing clinical disease). The decline in passive immunity in an infant is what dictates the recommended schedule of immunizations for infants.
Each lymphocyte has only one type of epitope receptor, but pathogens have many potential antigenic molecules, each of which may have several epitopes. In addition, the epitopes for some pathogens, such as those on influenza's hemagglutinen protein, change from year to year as a result of mutations. Consequently, the number of possible foreign epitopes is enormous, but the human genome only has about 30,000 genes. In view of this, how does the immune system manufacture all of the lymphocyte receptors needed to recognize so many different epitopes?
The answer is that this is accomplished through gene spicing. In most cases, a single gene encodes for a single protein.
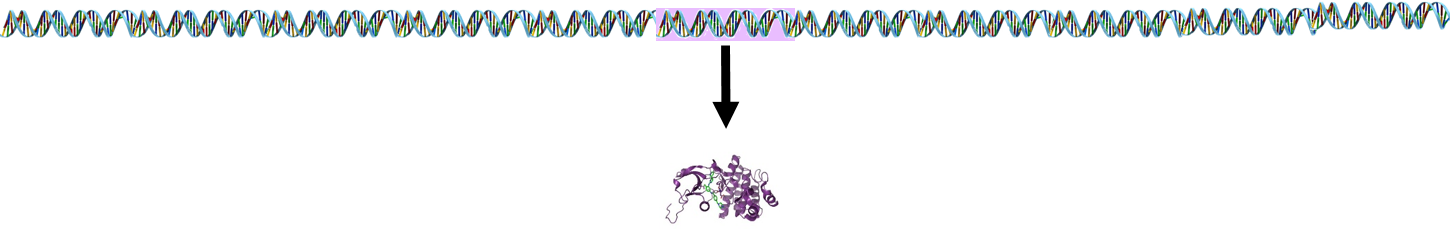
However, lymphocyte receptor proteins are produced by a different process. The stem cells that produce lymphocytes have a pool of 300-400 genes which encode for peptides in lymphocyte receptors. During development and maturation of lymphocytes, most of these genes are eliminated, and only 5-6 randomly selected genes are retained and spliced together. This spliced sequence encodes for receptors in the mature lymphocytes.
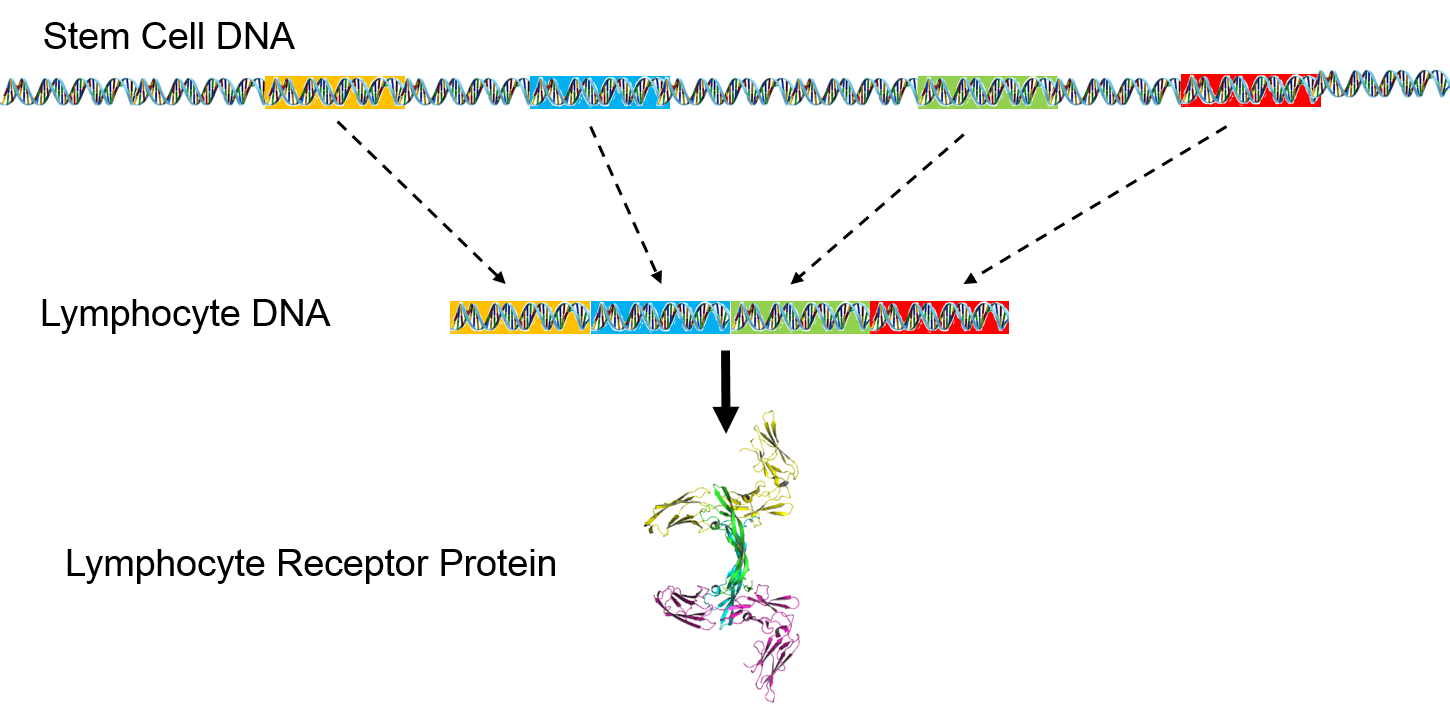
The pool of receptor genes in stem cells is like a deck of 300-400 cards. Each lymphocyte that is produced is dealt 5-6 of these at random, and the 5-6 genes can be shuffled into any order before they are spliced together. As a result, it is possible to synthesize a hundred million different receptors from the original pool of 300-400 genes. When it matures, each lymphocyte can manufacture just one type of epitope receptor.
This short video summarizes the process by which specific antibodies are made.
How does the immune system avoid attacking our own cells which also have potentially antigenic shapes? As a result of the random selection and splicing of 5-6 genes to create unique receptors, some of the lymphocytes created will have receptors that would bind our own proteins. However, during the maturation process in the thymus gland, those lymphocytes are induced to undergo apoptosis (programmed cell death), and they are permanently eliminated.
Autoimmune diseases, such as rheumatoid arthritis, lupus, & juvenile (type I) diabetes result when self-tolerance fails and lymphocytes attack our own cells.
The image on the rightbelow provides a brief summary of innate and adaptive immunity.
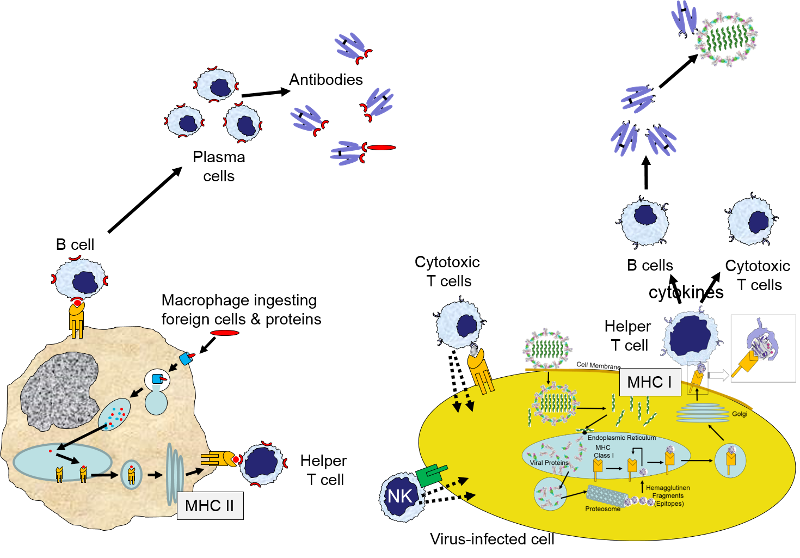
While not shown here, engulfment of a pathogen by a macrophage and damage to local cells would also trigger an inflammatory response, which would entail release of a variety of cytokines and chemoattractants, dilation of local blood vessels, and translocation of neutrophils, fluid, and complement proteins from capillaries into the affected tissue.
There are a number of conditions that cause deficient immune responses.
Inherited Immunodeficiency
Inherited immunodeficiency disorders can result in inability to produce antibodies, such as:
Acquired Immunodeficiency
Deficient immune response can also be the result of any number of acquired conditions, including:
There are several circumstances in which responses of the immune system are inappropriate, unwanted, or excessive. These include:
Allergies are undesirable inflammatory responses to environment exposures that are harmless to most people. A wide variety of substances can trigger an allergic reaction (referred to as allergens), including plant pollen, foods (e.g., peanuts, soy, seafood, eggs), animal dander (e.g., from cats, dogs or other furry animals), insect stings (e.g., bee sting allergy), mold, medicines. Some individuals are "atopic," meaning that they have an inherited predisposition to developing allergies. Exposure to an allergen in an allergic individual triggers the secretion of IgE antibodies, which trigger the release of histamine and other mediators from mast cells and basophils when the antibody binds to the allergen. The magnitude of the resulting inflammatory response can vary widely, ranging from mild to moderately severe local symptoms (e.g., allergic rhinitis/conjunctivitis, eczema or hives) to life-threatening conditions such as asthma and anaphylactic shock.
Autoimmune disorders are those in which an individual's immune system attacks its own tissues and organs. The underlying causes of autoimmune disease are not well understood, but one theory is that infections or other injuries to tissues cause alterations that confuse the ability of our immune system to distinguish between "self" and "non-self".
Common autoimmune disorders include:
and there are many other forms of autoimmune disease
As noted earlier, the MHC molecules are an means of distinguishing between "self" and "non-self". The MHC molecules in humans are referred to as the human leukocyte antigens (HLA antigens). The probability of transplant rejection is reduced when there is a close match between HLAs of donor and recipient. Rejection of transplanted tissue is mediated primarily by the adaptive immune system through cytotoxic T cells and B cells which differentiate into antibody-producing plasma cells.BB
Our red blood cells have glycoproteins that can be antigens. The four major "types" of glycoproteins on human red blood cells are classified as A and B, but the antigens on our red blood cells are co-dominant, meaning that one's blood type is determined by two alleles, neither of which is dominant. Therefore, the major blood types in humans are A (AA or AO), B (BB or BO), AB, and O. Another important antigen on red blood cells is the Rhesus or "Rh factor" which is classified as positive (+) or negative (-). Blood banks will match donor and recipient blood types to minimize incompatibilities, because transfusion of incompatible blood can result in destruction of the transfused blood cells and a sever transfusion reaction.
As described earlier in this module, cytokines are a large family of signaling molecules that play a vital role in orchestrating an inflammatory response and in ramping up the immune response to a pathogen. As a result, there are significant changes in blood vessels that allow proteins, fluid and cellular elements of the blood to leve the circulation and move into tissues to destroy foreign agents or substances. Cytokines are also responsible for activating cells of the immune system and stimulating lymphocytes to divide repeatedly and, in the case of B cells, to differentiate into antibody-secreting plasma cells. Most challenges by pathogens are resolved successfully, and the surge of immune response gradually subsides when the foreign agents have been contained and eliminated. Cytokine secretion subsides, and the large numbers of lymphocytes created through clonal selection also decline as a result of apoptosis. Only a relatively small number of memory cells are left. However, under some circumstances that are not well understood, the immune response does not subside normally. Instead, there is unbridled inflammation within tissues and key organs. Certain types of infection seem to carry a higher risk of cytokine storm, e.g., systemic infection and septic shock, SARS, bird flu, Hanta virus infection, and Ebola.
During the 1918 flu pandemic there was an unusually high death rate among healthy young people between the ages of 20-40. These appeared to be due to respiratory failure as a result of excess accumulation of edema fluid in the lungs as a result of an excessive inflammatory response. A similar phenomenon was noted during the H1N1 influenza pandemic in 2009.